Author: Minna E. Mathiasson
Institution: University of Maine
ABSTRACT
In Africa, stingless bees are greatly understudied organisms that possess incredible ecological and economic potential. Stingless bees are pollinators of tropical plant species and also yield medicinal hive products. Deforestation has caused the preferred habitat of stingless bees to become increasingly rare, forcing populations to relocate. Quality of environment has an immense impact on stingless bee biodiversity and behavior. Therefore it is important to study behavioral changes in different environments. This study aims primarily to explore changes in early colony development of an Afrotropical stingless bee, Hypotrigona sp., when introduced to a new habitat. Four colonies of Hypotrigona sp. were transferred from their original tropical rainforest habitat to a coastal savannah in Cape Coast, Ghana. Activity, colony development, and hive resource production were monitored over the course of four weeks. Regarding forage activity and division of labor, Hypotrigona sp. was found to develop similarly among the four replicates. Queen activity was greatly influential, while environmental factors such as temperature and relative humidity were found to be significant parameters for colony development. Acquiring this fundamental behavioral information is central to furthering conservation efforts and developing sustainable commercial management.
INTRODUCTION
Stingless bees (Hymenoptera: Apidae) belong to the tribe Meliponini and encompass a diverse population of eusocial bees found worldwide in tropical and subtropical regions. More than 400 species have been identified, 11 of which are found in Ghana (Kwapong, Aidoo, Combey, & Karkari, 2010). This study explores early colony development in a new habitat of a species of stingless bee within the genus Hypotrigona that has shown great ecological and economic potential due to its adaptability and abundance in Afrotropical regions.
Historically, indigenous communities have used stingless bees to increase their crop yields (Cockburn & Kwapong, 2013). Today, stingless bees remain an incredible pollination resource for both wild and cultivated crops. They are generalist flower visitors, key pollinators of nine crops, and partial pollinators of at least 20 others (Danaraddi, Hakkalappanavar, Birandar, Tattimani, & Vinod, 2007; Heard, 1999; Karikari & Kwapong, 2007). In addition, stingless beehive products—honey, bee pollen, and propolis—are medicinal and are known for their antibacterial and immunostimulatory properties. Unfortunately, meliponiculture is rare throughout all of Africa and honey is often harvested from natural colonies in the wild in an unsustainable manner, causing deleterious effects to both the colony and the environment (Sommeijer, 1999). Furthermore, the pollination services of Apis mellifera, the most globally common commercially managed bee for pollination and honey production, are declining due to disease, colony mismanagement, exposure to toxic crop chemicals and nutritional deprivation. In this time of insecurity, stingless bees have exceptional alternative potential (Karikari & Kwapong, 2007). As the only other social group in the Apidae family, stingless bees share many characteristics with honeybees, including social lifestyle in perennial colonies, nesting habits, and honey storage (Njoya, 2010). They are also the ideal pollinator for enclosed areas such as greenhouses due to their non-functional sting (Slaa, Sanchez, Sandi, & Salazar, 1999). Moreover, stingless bees promote habitat biodiversity due to their generalist foraging behavior (Karikari & Kwapong, 2007).
Deforestation and resource depletion in the natural habitat of stingless bees is a serious threat to their diversity (Dicks, Showler, & Sutherland, 2010). Stingless bees commonly nest in tree cavities, logs, or other crevices. However, they are forced to relocate when habitat and food sources diminish. Alternative nesting sites in keyholes, pipes, and other artificial environments do not allow colonies to expand and store appreciable amounts of products. Recent studies have shown that, unlike honeybees, which are more likely to be found in cultivated agricultural environments, stingless bees prefer forest habitats adjacent to cultivated areas (Banks et al., 2013). Therefore, studying stingless bees’ response to habitat change, especially during the rainy season when weather patterns are less predictable, is important for farmers intending to conventionally manage stingless bees.
Hypotrigona sp. constructs storage pots made of cerumen to store honey and pollen. They also construct cup-shaped brood cells that, unlike the progressively provisioned brood cells of honeybees, Apis mellifera, are mass provisioned with larval food by workers prior to oviposition into the cell by the queen and eventual sealing (Danaraddi et al., 2007). The larval food formula used by Meliponini species is a mixture of pollen, honey, and glandular secretions (Menezes, Vollet-Neto, & Fonseca, 2013). New cells are laid above sealed cells in a cluster and remain closed until the fully developed adult bee emerges (Njoya, 2009).
In the present study, we investigated the stages and rate of early colony development of Hypotrigona sp. in a new habitat. This study also aims to develop quality techniques to serve as a model for similar studies in other species of stingless bees that are less common and less adaptable. We hypothesized that the early colony development of Hypotrigona sp. would be affected by environmental changes, including temperature, relative humidity, new hives, unfamiliar habitat and forage, and removal of former hive products.
METHODS
Study Species
For this study, we chose the genus of stingless bee Hypotrigona because it is found in abundance throughout Afrotropical regions. Additionally, a recent study found Hypotrigona to be the most adaptable of four stingless bee genera reared in a laboratory setting (Njoya, 2009). In Ghana, Hypotrigona sp. is better known by its local names: Mimina (Akan) and Anihawmoa (Fanti).
Figure 1. Map of Ghana showing the location of Cape Coast in the Central Region.
Although the particular species of Hypotrigona remains unknown, it is certain that all of the bees in this study were of the same species due to their morphology and distinct entrance tube structure. Different species of Hypotrigona are difficult to distinguish and until very recently, no comprehensive report of stingless bees in Africa existed (Eardley, 2004). The various species of Hypotrigona can be differentiated based on morphological characters, structure of the hive entrance tube, and by geometric morphometric analysis (Combey, Galaschi Teixeira, Bonatti, Kwapong, & Francoy, 2013).
Colony and Site Selection
We removed four established colonies of Hypotrigona sp. from an original site at the International Stingless Bee Centre (ISBC) in Abrafo, Ghana (Figure 1). Hive entrances were plugged and the hives were brought approximately 30km south to the study site at Cape Coast (05° 07.115’ N, 001° 17.425’ W, elevation: 16 m). The study site was in proximity to blooming plant species including Albizia sp., Leucaena leucocephala, and Roystonea regia, all of which stingless bees are known to forage (Kajobe, 2008). The colonies chosen for the study had the following histories:
Colony A: Transferred to the ISBC on Feb. 28, 2011 from Bekawpa.
Colony B: Transferred to the ISBC on Nov. 17, 2009 from Bekawpa.
Colony C: Transferred to the ISBC on Nov. 17, 2009 from Bekawpa.
Colony D: Established as swarm from the ISBC on Feb. 13, 2012.
Colony Transfer and Set-Up
We transferred the bees from their original hives after a 24hr adjustment period in the new environment. All new hives were constructed out of plywood with interior dimensions at 13cm length x 8.5cm width x 8.5cm height. A glass panel covered by a wooden lid was placed over each hive to allow for observation. To prevent light from entering the hive, black electrical tape was used to seal the edges of the glass panel and lid. Honey and pollen pots present in the original hives were removed and weighed upon transferring the bees to the study hives.
The four study hives were placed on a table under a covered porch. The hives were arranged in the same orientation as the original hives at ISBC. Stingless beehives do not need to be widely distanced from one another; a recent study showed very high rejection rates in response to non-nest-mate odor in stingless bee populations so intrusion due to close proximity of hives is not an issue (van Zweden et al. 2010). The legs of the table were placed in containers of soapy water to deter predators such as lizards and ants.
Hive Entrance
Hive entrance development was monitored by daily measurements of the length of the entrance tube until construction ended. Color changes of the hive entrances were noted throughout the four weeks as this provides information on species identification and development prioritization.
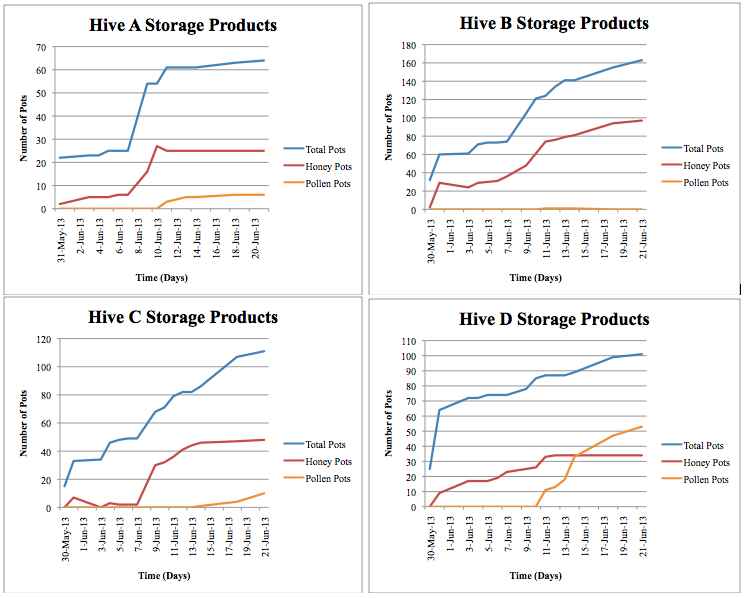
Figure 2. Individual colony storage products. ‘Total Pots” includes both unfilled and filled storage pots. “Honey Pots” includes honey pots filled and capped. “Pollen Pots” includes pollen pots filled over halfway with pollen.
Environmental Factors and Supplemental Feeding
Environmental factors influencing behavior, including relative humidity, temperature, and general weather patterns such as presence of sun or precipitation, were recorded daily during internal and external hive observations. The colonies were fed twice (Day 1 and Day 7) with a sugar syrup solution as a nectar replacement. They were also fed on Day 10 with their initial hive products (honey and pollen) that were removed from the colonies during the transfer. Bees were provided supplemental food resources for two reasons. Firstly, hive products were removed from the colonies during hive transfer so the bees were left without food stores. Secondly, the study was performed during the rainy season when foraging becomes more difficult. The food was made available in four small dishes outside of the hives.
When offered the initial hive products (honey and pollen pots removed during the hive transfer), the bees used only the honey and ignored the pollen and cerumen materials. There was a considerable increase in filled honey pots after the colonies were offered the hive products compared to other time points throughout the study (Figure 2).
Storage Pots and Products
The construction, arrangement, and filling of storage pots were monitored daily throughout the study. The total number of pots, number of unfilled pots, number pots filled with honey, and number of pots filled with pollen were recorded. At the end of the study, the volumes of ten honey pots from each of the four hives were calculated by measuring the three radii of the ellipsoid honey pot. The mean volume for all hives was calculated from the forty total pot volumes.
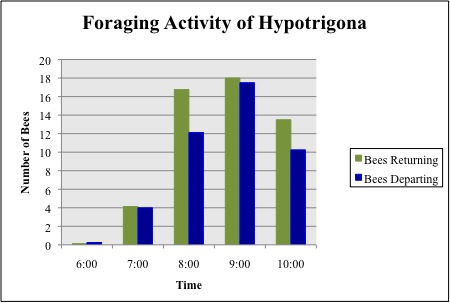
Figure 3. Mean number of returning and departing bees from the four colonies for a two-day period
Forage Activity
The time of day for peak forage activity was determined by counting the number of individual bees entering and departing from the hive during a 5min observation period. These measurements were taken independently for each hive for the first 20min of each hour, between 06.00 and 10.00. Forage activity measurements were taken over the course of two days, starting on Day 3 of the study. Peak forage activity during the first two days of observation occurred between the hours of 09.00 and 10.00 (Figure 3). As a result, observations were taken between these hours for the duration of the study. The data was plotted to find the peak time for forage activity of Hypotrigona sp.
External and Internal Hive Activity
During daylight hours, each colony kept five to seven guard bees stationed at of the outer edge of the entrance tube. The guards monitored bees departing from and returning to the hives. When a non-nest-mate attempted to enter the hive, the guards fought the intruder out of the hive entrance. Worker bees were observed coming and going from the hives during the morning and afternoon hours in suitable weather conditions. During nighttime hours, the guards abandoned their stations at the entrance tube. When activity was sensed near the hive (e.g. when a light was shone into the entrance tube), the guards quickly emerged.
Both internal and external hive activity was monitored during the daytime and nighttime in 5-minute internals for each hive. Daytime external observations were taken between the hours of 09.00 and 10.00 and daytime internal observations were taken between 10.00 and 12.00. Nighttime activity of each colony was monitored weekly between the hours of 21.00 and 22.00. Behavior and flight activity of guard bees were also recorded. Videos and photos were taken daily to further observe all activity.
Life Cycle, Division of Labor, and Brood Cell Development
The acceptance of individual roles, rules followed to specify tasks, coloration changes in individual worker bees, and queen-specific tasks were all noted over the course of the study. Videos and photos were taken daily to further observe all activities. The presence of a queen was confirmed through internal hive activity observations and her activity was monitored daily during hive observations for all of the colonies. The development of new brood cells and their arrangement was also observed.
Data Analysis
Data was analyzed using Microsoft Excel and Minitab® Statistical Software (Version 17; Minitab Inc., 2010). Mean bar (Figure 3) and individual line plots (Figure 2) were generated in Microsoft Excel. A two-way ANOVA and two-tailed t-test were run in Minitab® to analyze the relationship between environmental factors and the forage activity of Hypotrigona sp. (Figure 4). 95% confidence intervals are included in Figure 4.
RESULTS
Hive Entrance
Along with cleaning and sealing the new hive, building a hive entrance was among the first tasks completed by the bees after the transfer. All the colonies completed building the entrances within two or three days. The mean hive entrance length was 4.5mm.
Temperature and Humidity
We found a significant correlation between forage activity and environmental factors (Figure 4). Our results show that Hypotrigona sp. is more likely to forage in low humidity and high temperature conditions. A favorable range for peak foraging is temperatures above 29°C with relative humidity below 70% (Figure 4).
Figure 4. Forage activity represented by bars and primary axis. Environmental factors (temperature, relative humidity) are represented by lines and secondary axis. Values and standard errors represent an average of the four hives.
Storage Pots and Products
Two days after transfer, the colonies began constructing storage pots. All of the storage pots were similar in size and shape (ellipsoidal), and constructed of dark golden brown cerumen. The organization and distribution of honey and pollen pots varied between all hives. Pots were built near the hive entrances and along the walls in clusters. The mean volume of honey stored in an individual honey pot was 0.19mL. A sample calculation is shown here using the volume formula for an ellipsoid (variables a, b, and c represent the three radii of the ellipsoid). V=(4/3)πabc.
Each colony exhibited slightly different behaviors concerning storage products, and trends emerged in timing and progression (Figure 2). All colonies began to construct pots promptly upon transfer to the new hives. On the first day of observations, each colony had constructed between 15 and 35 storage pots. However, only 0 to 2 of these pots were filled with honey, while the rest remained empty. This suggests that the colonies waited until all the storage pots were constructed before beginning to fill any of them. The colonies all continued to build and fill new pots, always keeping empty pots available. The pots were always filled first with honey followed by pollen. Colonies A, B and D began to fill pollen pots on June 11. Colonies A and D stopped filling honey pots on this day and continued to increase their numbers of filled pollen pots until the end of the study.
External and Internal Hive Activity
Dead bees and debris were carried out of the hive and dropped just outside the entrance. The majority of cleaning activities took place during the first three days. The bees continued to clean throughout the course of the study during the morning hours only (before 10.00). The bees foraged for resinous materials to make propolis before they foraged for food supply. Within the first 36 hours of hive transfer, propolis was used to seal any imperfections inside the hives, the internal edges of the entrance tube, and the crack between the glass cover and the upper lid of the hive. All four hives had similar uses for propolis, though the quantity used varied between the colonies.
Daily, the bees built and filled storage pots, built and arranged brood chambers, and communicated in groups of three to seven, likely concerning the location of resources. The bees remained active during nighttime hours at a slightly slower pace.
Life Cycle and Division of Labor
Our observations show the age of Hypotrigona sp. can be determined by the color of the body. A newly hatched Hypotrigona sp. has an entirely light gray body. As the bee ages, the thorax darkens to black and the abdomen becomes golden. Ultimately, the entire body of the bee fully darkens to black.
The division of labor observed among the four colonies adhered to temporal polytheism, as is the case with most Hymenoptera. During the day, the younger Hypotrigona sp. workers remained in the hive. Though some older bees remained in the hive, most served as guards and foragers when conditions were appropriate. The queen’s primary roles were egg-laying and patrolling the brood cells.
Presence of Queen and Brood Cell Development
Queen activity was visible only for Colonies A and D. Every couple of days, the queen could be seen patrolling the brood cells in both hives. Two weeks after beginning observations, construction of new brood cells in these hives rapidly increased, continuing through the end of the study. The orderly formations of the brood cell clusters were very similar in Colonies A and D.
Colony C did not have a queen because she died during the colony transfer. Aside from the existing brood cells from the initial transfer, no new brood cells were constructed during the study and the presence of a replacement queen was never observed.
The queen in Colony B remained unobserved throughout the study. It is possible that she died during the colony transfer, but this was never visually confirmed. New brood cells were constructed in Colony B beginning at the same time as the other hives. The few cells that were constructed—less than ¼ of the number of cells constructed in Colonies A and D—were not distributed in the orderly formation observed in Colonies A and D.
Behavior was especially affected by the presence of an active queen. Though members of Colony C showed no sign of requeening, they did begin filling pollen pots at the end of the study, but showed no sign of new brood cells. Members of Colony B focused only on filling honey pots, and, at the end of the study, continued to display a daily increase in their number of honey pots. Bees residing in Colony B also built a small amount of brood cells, though they were arranged in a different pattern than those in Hives A and D (Figure 5). The inconsistency in brood arrangement in Colony B is likely due to the absence of a queen. Thus, worker bees probably laid eggs into the new brood cells. Worker oviposition, is a common trait of stingless bees, although it is quite rare in honeybees. By partially reactivating the hypopharyngeal glands, a colony of workers is able to rear brood (Cepeda, 2006).
DISCUSSION
This study affirmed that certain behaviors of Hypotrigona sp. are analogous to those of related insects. It also revealed new information about the bees’ colony structure, development, and characteristics in a new environment. The peak daily forage activity of Hypotrigona sp. found in this study (between 9.00 and 10.00) corresponded to that observed in studies of other stingless bee species (Biesmeijer & Tóth , 1998; Danaraddi et al., 2007; Kajobe & Kwapong, 2008; Nunes-Silva, Hilário, de Sousa Santos Filho, & Imperatriz-Fonseca, 2010). Differing weather conditions also affect the flight patterns of Hypotrigona sp. We found a significant correlation between foraging activity, temperature, and relative humidity. High temperatures and low relative humidity contributed positively to colony development because the bees were productive in their foraging efforts whereas periods of low temperatures and high relative humidity had a negative effect on development because the bees stayed in their hives and were therefore unable to forage. These relationships show the importance of environmental considerations in the development of meliponiculture in different environments.
Many stages of development aligned among the four replicates, such as the initial concentrated cleaning effort and transition into a routine morning removal of detritus from the hives. However, other studies have shown that meliponine workers do not rigidly assign tasks but rather adapt depending on the colony’s state (Cepeda, 2006). During this study, it was clear that individual behavior of Hypotrigona sp. was determined primarily by the needs rather than the size of the colony. This tendency for colonies to adapt based primarily on the colony’s state was exemplified by the timing and progression of the bees storing hive products and laying brood. The differences in development between the queenright Colonies A and D compared to Colony B, in which a queen was never seen, and Colony C, which lacked a queen, clearly demonstrated the highly positive influence that an active queen has on early colony development. The size of the colonies proved to be much less important than the presence of an active queen in regards to positive signs of colony development.
Figure 5. Comparison of new brood cell arrangements in Hives A (left) and B (right).
The removal of initial hive products and unfamiliar forage sites were additional stressors in the colonies’ adjustment to a new environment. Furthermore, tropical plants tend not to flower during the rainy season and typically do not flower continuously (Kajobe, 2008). The bees used the stored honey to provision foragers with energy during flight. A foraging behavior study indicated that altering forage sites slowed the reproductive fitness of stingless bee species (Kajobe, 2008). Therefore, providing the bees with a supplemental food resource such as a nectar substitute was critical. Supplemental feeding had a positive effect on the development of the colonies, and the number of filled honey pots in each hive increased after each feeding.
A possible limitation to this study is the fact that the species observed remains unknown. Species of Hypotrigona can be putatively differentiated based on structural and coloration differences in their hive entrances. Distinguishing characteristics of hive entrances include variation in length, relative flattening of the tube, and widening at the outer end of the entrance resulting in a trumpet shape, where the guards are positioned. The entrance tubes of the species of Hypotrigona used in this study were relatively short and had trumpeted ends. Morphological differences—facial and scutum features, wing venation, pubescence—in both males and workers are also used to classify individual Hypotrigona species. In addition to using the taxonomic references available for African stingless bees, molecular analysis must be employed to determine which species was present in our study. Although preliminary results indicate only one species was used, further analyses must be performed to confirm this.
Given that this was a preliminary study, the sample size was fairly small. However, in future studies, a larger sample size should be considered. Additionally, future studies should focus on development over a longer period of time and in multiple environments. As this was the first study conducted using Hypotrigona, there are currently no existing behavioral data with which to compare these results. Future studies should obtain information on early colony development, foraging, feeding, and storage behavior of Hypotrigona sp. in their original rainforest habitat to enable a comparative analysis. This study offers information on monitoring important factors that indicate differences in development, such as queen activity and weather variation. It also provides insight into the behavior of stingless bees and can provide a methods baseline for similar studies on less adaptable species of stingless bees.
Conclusion
An interspecific study was conducted on the early colony development of Hypotrigona sp. with the intention to expand the limited knowledge of the nesting ecology of Afrotropical stingless bee populations and the effects that new habitat may have on early colony development. The central question addressed the stages and rate of Hypotrigona sp. early colony development and how it is affected by certain factors such as environmental changes; new hives; unfamiliar habitat and forage; and the removal of former hive products. The activity of Hypotrigona sp. was shown to be driven primarily by environmental conditions. Peak forage activity was found to occur between 09.00 and 10.00, and optimal weather conditions significantly increased bee activity. High temperature and low humidity contributed positively to development. In addition to environmental factors, the presence of a queen and supplemental feeding greatly influenced the state of the colony, which, in turn, affected the individual colony’s progression of storing hive products and constructing brood chambers.
The services of stingless bees are critical to the wellbeing of both Afrotropical environments and of human populations due to the crops dependent on bee pollination. Developing strategies to ensure the health of stingless bees requires insight into their habits, such as the behavioral knowledge gained from this study. Given the great importance of stingless bees in their native regions, there is a need to develop quality habitat management technique to ensure their survival and reduce population decline.
ACKNOWLEDGEMENTS
Much gratitude is extended to Peter Kwapong for supervising this project and for providing advice and instruction for carrying out the study and analysis of the data collected. An additional thanks is given to Daniel A. Wubah (DAW) as program director of the Virginia Tech-UCC REU program. This project was supported with funds provided by the National Science Foundation (DBI-1000650).
REFERENCES
Aidoo, K., Kwapong, P., Combey R., and Karikari A. (2011). Stingless bees in Ghana. Bees for Development Journal 100, 10-11.
Banks, J.E., Hannon, L., Dietsch, T., Castro, S., Urena, N., and Chandler, M. (2013). Effects of proximity to forest habitat on hymenoptera diversity in a Costa Rican coffee agroecosystem. The Pan-Pacific Entomologist, 89(1), 60-68.
Biesmeijer, J.C., and Tóth, E. (1998). Individual foraging, activity level and longevity in the stingless bee Melipona beecheii in Costa Rica (Hymenoptera, Apidae, Meliponinae). Insectes Sociaux, 45(4), 427-443.
Cepeda, O.I. (2006). Division of labor during brood production in stingless bees with special reference to individual participation. Apidologie, 37, 175-190.
Cockburn, C.L., and Kwapong, P.K. (2013). Shelf-life and variances in antimicrobial properties of honey from Meliponula bocandei and Meliponula ferruginea in central Ghana. Journal of Young Investigators, 25(1), 10-14.
Combey, R., Galaschi Teixeira, J.S., Bonatti, V., Kwapong, K., and Francoy ,T.M. (2013). Geometric morphometrics reveals morphological differentiation within four African stingless bee species. Annals of Biological Research, 4(11), 93-103.
Danaraddi, C.S., Hakkalappanavar, S., Biradar, S.B., Tattimani, M., and Vinod, S.K. (2011). Studies on foraging behaviour of stingless bee, Trigona iridipennis Smith at Dharwad, Karnataka. International Journal of Forestry and Crop Improvement, 2(2), 163-169.
Dicks, L.V., Showler, D.A., and Sutherland, W.J. (2010). Bee conservation: Evidence for the effects of interventions. Exeter, United Kingdom: Pelagic Publishing.
Eardley, C.D. (2004). Taxonomic revision of the African stingless bees (Apoidea: Apidae: Apinae: Meliponini). African Plant Protection, 10(2), 63-96.
Heard, T.A. (1999). The role of stingless bees in crop pollination. Annual Review of Entomology, 44, 183-206.
Kajobe, R. (2008). Foraging behaviour of equatorial Afrotropical stingless bees: habitat selection and competition for resources (Doctoral dissertation). Retrieved from Utrecht University Repository.
Karikari, A.S., and Kwapong, P.K. (2007). A survey of indigenous knowledge of stingless bees (Apidae: Meliponini) in the central region of Ghana. Journal of the Ghana Science Association, 9(2), 132-137.
Kwapong, P., Aidoo, K., Combey, R., and Karikari, A. (2010). Stingless Bees: Importance, Management, and Utilisation. Accra, Ghana: Unimax Macmillan Ltd.
Menezes, C., Vollet-Neto, A., and Fonseca, V.L.I. (2013). An advance in the in vitro rearing of stingless bee queens. Apidologie, 44, 491-500.
Minitab 17 Statistical Software (2010). [Computer software]. State College, PA: Minitab, Inc.
Njoya, M.T.M. (2010). Diversity of stingless bees in Bamenda Afromontane forests – Cameroon: nest architecture, behaviour and labour calendar(Doctoral dissertation). Retrieved from Bonner Dissertationen und Habilitationen online.
Nunes-Silva, P., Hilário, S.D., de Souza Santos Filho, P., and Imperatriz-Fonseca, V.L. (2010). Foraging Activity in Plebeia remota, a Stingless Bees Species, Is Influenced by the Reproductive State of a Colony. Psyche, 2010.
Slaa, E.J., Sanchez, L.A., Sandi, M., and Salazar, W. (1999). A scientific note on the use of stingless bees for commercial pollination in enclosures. Apidologie, 31, 141-142.
Sommeijer, M. (1999). Beekeeping with stingless bees: a new type of hive. Bee World, 80(2), 70-79.
van Zweden, J.S., and d’Ettorre, P. (2010). Nestmate recognition in social insects and the role of hydrocarbons. In Blomquist, G.J., and Bagnéres, A.G. (Eds.), Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology(222-243.) Cambridge, United Kingdom: Cambridge University Press.