Author: Jordan Hall
Institution: Duke University
Date: August 2012
You can’t teach an old cell new tricks — or so we thought. Similar to those lazy pets stuck in their ways, we tend to think of cells as static entities. A research article published online in Cell Stem Cell in June, however, suggests otherwise. In this study, researchers from the Gladstone Institutes in San Francisco and University of California San Francisco successfully converted human skin cells into functional brain cells — using only a single genetic factor. This research offers new avenues for repairing the brain as well as testing therapeutic drugs and compounds on lifelike model systems.
Over the past couple of years, scientists have demonstrated similar transformations. In 2010, Dr. Shinya Yamanaka, a pioneer of the cellular reprogramming field also from the Gladstone Institutes, successfully transmitted four specific genetic factors into mouse and human skin cells to revert them into a stem cell state. These embryonic stem cells, or pluripotent stem cells, can adopt any cellular identity. They pose an inherent danger, however, in that these rapidly dividing stem cells can form tumors when used to repair or replace tissue.
Following the lead of this advancement, other research groups used viruses to import outside transcription factors, which are proteins that help ‘turn on’ genes and other small molecules to transform human skin cells directly into functional nerve cells. This approach bypasses the lengthy and precarious middle step of forming pluripotent stem cells.
In the current study, Dr. Yadong Huang and his team have further improved the method: “here, we present a novel method for generating self-renewable, multipotent, neural lineage-restricted, and nontumorigenic [neural stem cells] from mouse and human [skin cells] by direct reprogramming with one factor,” exclaims Dr. Huang.
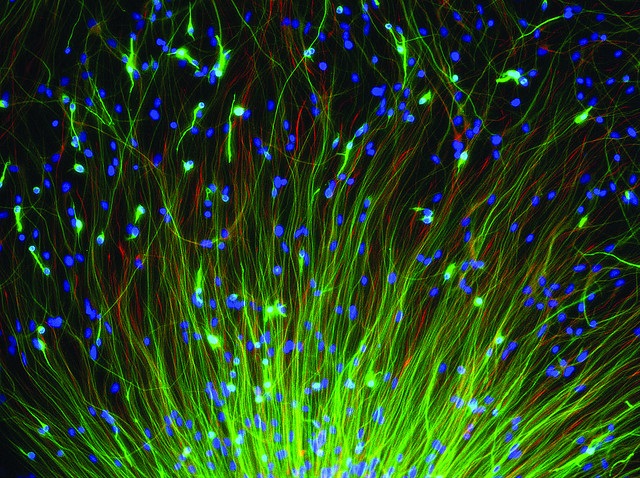
The researchers began with a trial-and-error experiment to identify the most important reprogramming factors. After choosing five transcription factors important for producing and maintaining neural stem cells, they then used viruses to transmit different combinations of these factors to mice skin cells. Interestingly, application of just a single transcription factor – Sox2 –was most efficient.
When taken into the skin cells, Sox2 prodded the cells to change their physical characteristics. For cells, their physical shape largely dictates their function, and thus these morphing skin cells were ultimately changing their identity. Within just two days, the skin cells had become induced neural stem cells (iNSCs). Capable of self-renewal, these iNSCs quickly formed colonies that expressed molecular markers identical to embryonic neural stem cells. A huge advantage over the previous method, these iNSCs could develop into multiple types of functional nerve cells, given the appropriate environment. The team then applied the same formula to human skin cells and achieved similar results.
This research is revolutionary in that it provides a straightforward means of generating many types of functional brain cells. “Having a patient-derived population of multipotent iNSCs would bypass some of the disadvantages of pluripotent and terminally differentiated cell populations,” explains Dr. Huang. Further, he envisions two major applications for this strategy: “our Sox2-reprogrammed iNSCs can serve as a model system for unveiling disease pathogenesis, for drug screening and toxicity tests, and ultimately for cell transplantation therapies.”
As mentioned in the empirical paper, Dr. Huang and his team are currently testing different molecular compounds on the iNSCs to optimize the production of each type of brain cell.
As Dr. Huang envisions, “[if] we can pinpoint which genes control the development of each neuron type, we can generate them in the petri dish from a single sample of human skin cells...[and] then test drugs that affect different neuron types - helping us to put drug development for neurodegenerative diseases on the fast track.”
This science news brief was written under the guidance of JYI Science Writing Mentor Jake Berkowitz.