Author: Alexandra B. Bentz
Institution: Appalachian State University
Abstract
Flavonoids are natural antioxidants derived from plants and commonly found in foods, such as fruits and vegetables, with the ability to sequester free radicals. Quercetin, part of a subclass of flavonoids called flavonols, has received considerable attention because of its overwhelming presence in foods. Quercetin is believed to protect against several degenerative diseases by preventing lipid peroxidation. However, the degree and method of quercetin's absorption in vivo has yet to be absolutely determined. It is thought that the predominant glucoside form is converted to the aglycone, which is then converted to one of several quercetin metabolites. Synthesis and study of quercetin metabolites is key to understanding how quercetin acts as an antioxidant. This review provides a survey of the literature regarding flavonoids in general, and more specifically, quercetin. The structure and corresponding antioxidant properties are discussed to emphasize the importance of flavonoids. However, the antioxidant capability of flavonoids expressed in vitro may prove to be a moot point as their degree of absorption is controversial. Several of the studies performed in vivo have offered the alternative view that better absorbed antioxidants, like vitamin C, are exerting the antioxidant effect witnessed after consumption of certain food, such as apples. This review addresses this issue by providing a survey of in vitro and in vivostudies regarding the absorption, bioavailability, and antioxidant properties of flavonoids, specifically quercetin and its metabolites.
Introduction
Free radicals are reactive molecular species with unpaired electrons that oxidize other molecules to gain electrons and stabilize themselves. The reaction produces another free radical, initiating a domino effect of free radical stabilization and formation (Machlin and Bendich 1987). Free radicals can oxidize macromolecules, such as DNA, proteins, carbohydrates, and lipids (Uddin and Ahmad 1995). Free radical damage can cause unsaturated bonds in membrane lipids to lose fluidity when peroxidized and proteins to denature (Machlin and Bendich 1987). The oxidative damage created by free radicals is referred to as oxidative stress, and has been associated with several degenerative diseases, including cardiovascular and inflammatory diseases, cancer, aging, and stroke (Machlin and Bendich 1987; Uddin and Ahmad 1995).
Free radicals such as superoxide, hydrogen peroxide, and hydroxyl, are produced in the cells and the environment. Within cells, oxidative metabolism generates and propagates free radicals, especially when transition metal ions (e.g., iron and copper) aid in electron transfer (Uddin and Ahmad 1995). Free radicals also occur from environmental sources including tobacco smoke, air pollutants, anesthetics, pesticides, and radiation (Machlin and Bendich 1987).
Many organisms use oxygen during metabolism, and can thus be subjected to oxidative stress. However, physiological mechanisms exist to hinder free radical formation. For example, transition metal ions are bound to proteins to limit their catalytic involvement in free radical formation, while molecular oxygen is bound to specific enzymes, which regulate its reduction to superoxide (Uddin and Ahmad 1995). Plants have also developed methods of stopping free radical damage. Secondary metabolites, called flavonoids, have a polyphenol structure, which contains numerous double bonds and hydroxyl groups that can donate electrons through resonance to stabilize the free radicals (Machlin and Bendich 1987). The radical scavenging properties associated with the structure of flavonoids defend against oxidative stress and in doing so, reduce heart disease, prevent cancer, and slow the aging processes in cells responsible for degenerative diseases (Hollman and Katan 1997).
There are several subclasses of flavonoids: flavanols, flavanones, flavones, isoflavones, anthocyanidins, and flavonols (Hollman and Katan 1997). The divisions in flavonoid subclasses are based on structural properties. The plants, and thus foods they are found in differ, as well. The flavanols are found in red grapes and red wine, flavanones are in citrus foods, flavones are in green leafy spices, isoflavones are found in soy foods, anthocyanidins are in berries, and flavonols are found in almost all foods (Beecher 2003).
Quercetin, a major representative of the flavonol subclass, has received considerable attention. Quercetin and its sugar-bound, or glucosylated, forms represent 60-75% of flavonoid intake (Bouktaib et al. 2002). Quercetin has displayed the ability to prevent the oxidation of low-density lipoproteins (LDL) by scavenging free radicals and chelating transition metal ions. As a result, quercetin may aid in the prevention of certain diseases, such as cancer, atherosclerosis, and chronic inflammation (Hollman and Katan 1997; Murota and Terao 2003). This review will provide a brief introduction to flavonoid structure to complement their action as antioxidants, the benefits of which will be discussed to convey the importance of flavonoids. However, the controversy surrounding the absorption of flavonoids, such as quercetin, will be the main focus. There is a need to determine the degree of absorption and bioavailability of flavonoid metabolites in order to effectively defend against the notion that other antioxidants, like vitamins, found in foods along with flavonoids are actually exerting the antioxidant effect.
Structure
The basic flavonoid structure consists of two phenyl groups joined by a three carbon bridge (Bohm 1998; Beecher 2003). Flavonoids are divided into two main classes, those in which the three-carbon bridge is "open" and those in which the three-carbon bridge is involved in a heterocyclic ring, referred to as ring C (Figure 1)(Bohm 1998; Beecher 2003). Variations in ring C and the various substitution patterns available for rings A and B allow for a variety of flavonoid structures (Hollman and Katan 1997).
Figure 1. Basic Flavonoid Structures (Redrawn from the source)
Many of the subclasses of flavonoids vary by the functional group placed on ring C. Flavanol and anthocyanidins are the only two subclasses that do not have a 4-oxo group, but they do contain a 3-hydroxyl group along with the flavonols. The flavones, isoflavones, and flavonols have a 2-3 double bond on ring C, and anthocyanidins have a 1-2 and 3-4 double bond. The C ring on the subclasses is joined to ring B on carbon 2, except for isoflavones which are joined at carbon 3. These various substitution patterns not only define the subclasses, but also affect their absorption and their ability to act as antioxidants (Beecher 2003).
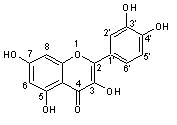
When the flavonol quercetin (3,5,7,3',4'-pentahydroxyflavone) (Figure 2)
Figure 2. Quercetin
reacts with a free radical, it donates a proton and becomes a radical itself, but the resulting unpaired electron is delocalized by resonance, making the quercetin radical too low in energy to be reactive (Mariani et al. 2008). Three structural groups aid in quercetin's ability to maintain its stability and act as an antioxidant when reacting with free radicals: the B ring o-dihydroxyl groups, the 4-oxo group in conjugation with the 2,3-alkene, and the 3- and 5-hydroxyl groups (Hollman and Katan 1997). The functional groups can donate electrons to the rings, which increase the number of resonance forms available in addition to those created by the benzene structure (Mariani et al. 2008).
Many flavonoids are bound to sugars in their natural state, the O-glycoside form, where glycosylation can occur at any hydroxyl group to yield a sugar. The most common quercetin glycosides have a sugar group at the 3-poistion, such as quercetin-3-O-β-glucoside (Figure 3) . It is these glycosylated structures that are most common in nature, not the aglycone, or parent compound (Murota and Terao 2003).
Figure 3. Quercetin-3-O-ß-glucosideSynthesis
The biosynthesis of phytochemicals, like flavonoids, is a defensive response of plants to their environment. Flavonoids often function as protection from ultraviolet sunlight and lipid peroxidation (Mariani et al. 2008). Mohle et al. (1985) demonstrated that when dill cell cultures were subjected to UV-B radiation, the predominant flavonoid synthesized was quercetin-3-O-β-glucuronide. They proposed that the biosynthesis of flavonoids is regulated by ultraviolet light and their accumulation acts as a defense.
Most studies assessing the antioxidant properties of quercetin utilize the aglycone form; however, analysis of plasma after quercetin consumption indicates that quercetin metabolites, like glucuronide (quercetin-3-O-β-D-glucuronide), are the primary compounds circulating in the blood (Bouktaib et al. 2002). The metabolites are also what are primarily found in plants (Mohle et al. 1985). The aglycone is used in studies because there are few quercetin metabolites commercially available. The chemical synthesis of the metabolites, however, is possible (Figure 4) (Bouktaib et al. 2002).
Figure 4. Synthesis of quercetin-3-O-ß-D-glucuronide (Redrawn from the source)
Bouktaib et al. synthesized quercetin-3-O-β-glucuronide (Figure 4) from quercetin aglycone (compound 1) by starting with the selective protection of the catechol on ring B with dichlorodiphenylmethane to produce a ketal (compound 2). Compound 2 was glucosylated at the hydroxy group in the 3-position and reacted with potassium carbonate in dimethylformamide to give compound 3. Compound 3 was then reacted with benzyl bromide in DMF and K2CO3 to protect the remaining hydroxyl groups. Deprotection of the acetoxy groups was carried out by sodium methoxide with neutralization by anionic resin in the protonated form. Compound 4, quercetin-3-glucoside, was oxidized by NaOCl in the presence of NaBr, and catalyzed by 2,2,6,6-Tetramethylpiperidine-1-oxy (TEMPO) to get glucuronic acid (compound 5). Phase transfer catalysis was used to overcome the low solubility of the protected quercetin glucoside while thin layer chromatography indicated that the glucoside was consumed after 2 hours. Ethyl acetate precipitated the glucuronide, which was purified by column chromatography on a silica gel. Final purification was performed on a LC-18 SPE cartridge and a methanol/water mixture was used as the eluent to obtain pure quercetin-3-O-β-D-glucuronide (Bouktaib et al. 2002).
Availability within Food
Researchers have begun to identify and quantify flavonoids from various foods. D'Abrosca et al. (2007) compared the flavonoid content of the flesh and peel of apples finding the highest concentration of flavonoids in the peel. Rietveld and Wiseman (2003) studied green and black tea, demonstrating that they both contained approximately 200 mg/cup for a typical brew. In 1996, Hollman et al. demonstrated the flavonoid content in various vegetables, fruits, and beverages. Hollman and co-workers hydrolyzed the glycoside form extracted from plants to determine the parent compound, and performed high-performance liquid chromatography (HPLC) to separate and quantify the flavonols. The highest concentrations of flavonols were found in vegetables like onions and broccoli, fruits like apples, cherries, and berries, and drinks such as tea and red wine. A more extensive list of quercetin aglycone content in foods, as determined by HPLC, was composed by the United States Department of Agriculture (Table 1) (USDA 2003).
Table 1. Quercetin Content in Selected Foods
Food preparation and storage can affect quercetin concentrations in food. Foods that have been fried and boiled show lower quercetin content. Boiling causes the greatest decrease in quercetin due to thermal degradation and the leaching action of boiling water (Ahearn and O'Brien 2002). However, this varies between foods, such as onions because they contain quercetin conjugates that can retain their stability up to temperatures of 100oC. The quercetin content in foods is also dependent on storage effects. Onions can lose 25-33% of their quercetin content in the first twelve days of storage, but suffer minimal losses thereafter (Beecher 2003). In contrast, the quercetin level in strawberries has been shown to increase approximately 32% when stored at -20oC for nine months. The process by which the foods are grown is also pertinent. For example, flavonol content is higher in plants exposed to higher levels of ultraviolet-B rays due to their action as possible defense mechanisms against the UV light. Plants grown in the United Kingdom were found to have a lower flavonol content than those grown in South Africa because of the use of greenhouses, which block the ultraviolet-B rays (Ahearn and O'Brien 2002).
The major dietary sources of flavonols vary with geography and culture. Red wine is the predominant source in Italy and tea is the major source in the Japanese and Dutch cultures. In the U.S., Finland, Greece, and former Yugoslavia, onions and apples are the primary dietary source of flavonols (Hollman and Katan 1997). The average total flavonoid intake for an individual in a day ranges from 24mg/d in Finland to 73 mg/d in Holland (Table 2)
Table 2. Flavonoid consumption in select countries
(Beecher 2003). A high intake of isoflavones is consistent with the Japanese culture, as their diets are high in soy foods, while tea, a source of flavanols, is popular in Holland. The average daily intake of flavonols ranged from 4mg/d in Finland to 30 mg/d in Denmark (Beecher 2003).
Antioxidant Properties
Quercetin is considered to be a strong antioxidant due to its ability to scavenge free radicals and bind transition metal ions. These properties of quercetin allow it to inhibit lipid peroxidation (Hollman and Katan 1997; Sakanashi et al. 2008). Lipid peroxidation is the process by which unsaturated fatty acids are converted to free radicals via the abstraction of hydrogen (Figure 5) (Young and McEneny 2001). The subsequent free radicals are oxidized by molecular oxygen to create lipid peroxy radicals. This process is propagated by the resulting lipid peroxy radicals extracting hydrogen from other unsaturated fatty acid molecules to create more free radicals. It is catalyzed, in part, by the presence of trace amounts of transition metal ions. Lipid peroxidation can create deleterious effects throughout the body, such as cardiovascular and neurodegenerative diseases; however, it can be terminated by antioxidants, like quercetin, which interfere by reacting with the radicals formed (Kahl and Hildebrandt 1986; Hollman and Katan 1997; Balazs and Leon 1994).
Figure 5. Lipid peroxidation (Redrawn from the source)
The oxidation of low-density lipoproteins (LDL) can result in the formation of atherosclerotic plaques, leading to cardiovascular disease (Hollman and Katan 1997). However, several studies have illustrated quercetin's ability to inhibit LDL oxidation. Graf and co-workers found a 21% reduction in cardiovascular disease mortality when the intake of quercetin was greater than 4mg/d (Graf et al. 2005). Chopra et al. (2000) gave one group of males 30mg/d of quercetin and another group 1g of red wine powdered extract for two weeks, prior to which there had been a placebo period for all participants so that each could be their own control. The red wine extract was in the form of a powder and contained several flavonoids, among which quercetin constituted 3.5 mg per gram of powder. Every participant was required to keep a journal of their intake of specific foods, including: fruits, vegetables, chocolates, fruit juices, milk, and alcohol. Vitamins C and E plasma concentrations were also measured along with flavonoids. They reported that the red wine extract and quercetin inhibited LDL and there was no effect on plasma concentrations of vitamin C and E. However, plasma concentrations of LDL remained constant. Chopra and co-workers suggested that LDL-cholesterol is only lowered by quercetin in hyperlipidemic patients; otherwise, quercetin inhibits LDL oxidation (Chopra et al. 2000).
The vulnerability of brain lipid membranes to lipid peroxidation is thought to lead to neurodegenerative disease, such as Alzheimer's and Parkinson's disease (Balazs and Leon 1994). Balazs and Leon (1994) found that oxidative stress occurring in the brain membrane lipids is associated with the extracellular accumulation of amyloid beta-peptide, which precedes neural losses in Alzheimer's patients. Yet, formation of amyloid plaques can be prevented by taking antioxidants (Ansari et al. 2008; Harman et al. 1976). In this situation, quercetin does not only stop the propagation of lipid peroxidation, but also increases glutathione (GSH) levels (Ansari et al. 2008). GSH is part of the neuron's defense against oxidative damage. When the superoxide radical is formed, the radical can be converted to the hydrogen peroxide radical by superoxide dismutase; however, GSH can convert hydrogen peroxide to oxygen and water, preventing the formation of free radicals (Balazs and Leon 1994).
Quercetin can also reduce inflammation by scavenging free radicals. Free radicals can activate transcription factors that generate pro-inflammatory cytokines, which are often found elevated in patients that suffer from chronic inflammatory diseases (Boots et al. 2008). Chronic prostatitis is not well understood, but it is thought that the disease inflammates the genital tract. Alexander et al. (1998), examined semen samples taken from normal men and men with chronic prostatitis, measuring the levels of the cytokines tumor necrosis factor-alpha and interleukin-1 beta. The results showed that men with the disease had higher levels of both pro-inflammatory cytokines in seminal plasma. In another study, Shoskes and co-workers administered men with chronic prostatitis 500mg of quercetin twice a day for one month. As a result, 67% of the men had a 25% improvement in symptoms. In 1999, Shoskes et al. gave men with the disease quercetin and Prosta-Q (bromelain and papain), which increases absorption of quercetin, finding that 82% had at least a 25% improvement.
Oxidative stress can cause cell death by means of prolonged elevations of intracellular Ca2+concentrations (Sakanashi 2008). Elevated levels of Ca2+ concentrations lead to an increase in energy expenditure and subsequent initiation of cytoskeletal degradation, which can lead to strokes and acute neuronal losses (Nicoteral and Orrenius 1998). However, quercetin can protect cells suffering oxidative stress and thus prevent Ca2+-dependent cell death (Sakanashi 2008). In a 15 year study following 550 middle-aged men, those with a flavonol intake greater than 30mg/d had a 60% reduction in their risk for strokes (Hollman and Katan 1997).
Quercetin can also protect against the more obvious environmental causes of free radicals, such as smoking. Cigarette tar is a source of free radicals, which has been found to damage erythrocyte membranes. Begum and Terao (2002) found that the quercetin aglycone and its conjugate metabolites (quercetin-3-O-β-glucuronide and quercetin-3-O- β -glucoside) could protect erythrocytes from the membranous damage that is caused by smoking. The control used in the study was flavone, which has the basic structure of quercetin but no hydroxyl groups, and it had no effect on the erythrocytes. This indicated that the hydroxyl groups are important to the antioxidant properties of quercetin.
Bioavailability
The antioxidant ability of flavonoids is assumed to arise from its structure, but whether these effects occur within the body is questionable because little is known about their bioavailability. Flavonoids are thought to be poorly absorbed because the naturally occurring glycosides' sugar moieties elevate the molecules' hydrophilicity, and no enzyme is known to split the glycosidic bond. Aglycones, sugar-free flavonoids can efficiently pass through the gut wall, but flavonoids are rarely found as aglycones in plants. It has been suggested that the colon has microorganisms that can hydrolyze the glycosidic bond, creating the aglycone, but the process also degrades the compound (Murota and Terao 2003; Hollman et al. 1997).
It is generally thought that the flavonoid glycosides enter the colon and are hydrolyzed to the aglycone by enterobacteria. The aglycone is then absorbed in the large intestine easily because of its lipophilicity, and then metabolized in the liver by O-methylation, glucuronidation, and/or sulfation. The quercetin aglycone can act as a pro-oxidant, thus converting the aglycone to the metabolites may be helpful in avoiding the harmful effects, and the metabolites have been shown to retain their antioxidant properties. Quercetin glucosides are able to pass through the epithelial cell layer, but they have a lower efficiency than the quercetin aglycone. Therefore, the hydrolysis of the glucoside to the aglycone accelerates the absorption of quercetin (Murota and Terao 2003).
Yet, many studies analyzing the bioavailability of flavonoids and quercetin have shown contradictory results. Hollman et al. (1995) suggested that the absorption of the glucoside is better than the aglycone, due to the actions of the glucose transporter (SGLT-1). Yet, another study showed that quercetin-4'-O-β-glucoside was absorbed more effectively than quercetin-3-O-β-glucoside or quercetin-3,4'-diO-β-glucoside because quercetin-4'-O-β-glucoside is more lipophilic than the other quercetin glucosides (Murota and Terao 2003). The studies suggest that quercetin absorption depends on the variety and position of the sugar groups attached. In 2000, Walle et al. (2000) found that the quercetin glucosides were completely hydrolyzed to their aglycone form before intestinal absorption, supporting the more widely accepted idea.
The quercetin aglycone or glucoside is not found in human plasma, however, quercetin conjugates, such as quercetin-3-glucuronide, quercetin-3'-sulfate, and isorhamnetin-3-glucuronide do occur in human plasma (Janisch 2004). Day et al. (2001) performed a study in which they demonstrated that 1.5hrs after consumption of onions, the glucoside and aglycone form of quercetin were not present in human plasma, but rather the metabolites quercetin 3'-sulfate and quercetin-3-glucuronide. Wittig et al. (2001) also demonstrated that neither the quercetin aglycone nor the glucoside was detected in human plasma after consumption of fried onions; rather, they found five different quercetin glucuronides. In a study performed by Moon et al. (2001), rats were feed quercetin and two quercetin conjugates were discovered in the plasma; quercetin-3-O-β-D-glucuronide and quercetin-4'-O-β-D-glucuronide. On further testing, quercetin-3-O-β-D-glucuronide possessed the ability to act as an antioxidant on copper ion-induced oxidation of human plasma LDL and showed radical scavenging properties, showing that the conjugates can act as antioxidants (Moon et al., 2001).
It has been proposed that flavonoids, like quercetin, do not necessarily need to be absorbed to exert an effect. After researchers fed teas and wines (both sources of quercetin) to rats, high concentrations of flavonoids were found on the gut lumen of the rats, resulting in a reduction of oxidative damage to DNA in caecal mucosal cells (Scalbert et al. 2002). This study would suggest that some dietary flavonoids can exert a positive effect regardless of their poor absorption.
The dietary intake of quercetin usually coincides with the ingestion of the other compounds in the same food source, but little research has been done concerning how this affects quercetin's absorption. One study by Azuma et al. (2002) discovered that the simultaneous ingestion of lipids (e.g., lecithin, fish oil, and beef tallow) and emulsifiers (e.g., sodium caseinate and sucrose fatty acid ester) with quercetin increased the accumulation of quercetin metabolites in the blood plasma.
The degree to which flavonoids can be absorbed has yet to be unanimously agreed upon. As a result, some studies have attributed the antioxidant effects of certain flavonoid-containing foods to other compounds. One study proposed that the increased antioxidant effects measured after apple consumption could result from fructose; volunteers that consumed fructose at levels equal to those found in apples also demonstrated increased antioxidant capability of plasma. The fructose treatment led to an increase in plasma urate, a metabolic antioxidant. Therefore the researchers suggested that the antioxidant effect was a result of the increased levels of urate, not the flavonoids present in apples (Lotito and Frei 2004).
This counter-argument has primarily arisen from flavonoids' low absorption compared to other antioxidants found in foods, like vitamin C. The plasma concentration typically found in humans after consumption of foods containing flavonoids is between 0.06 and 7.6 μM, this is also the range for quercetin. Vitamin C reaches levels between 30150 μM (Lotito and Frei 2006). Pedersen et al. (2000) reported that after volunteers consumed blueberry juice, cranberry juice, or sucrose as a control, plasma antioxidant capacity only increased after consumption of cranberry juice. The primary flavonoids in cranberries are anthocyanidins and flavonols; however, the largest concentration increase in the plasma was accomplished by vitamin C. The blueberries had no effect and they attributed this to the low vitamin C levels (Pedersen et al. 2000). These results in support of the counter-argument against flavonoid antioxidant capacity in vivo indicate the importance of experimental design and the significance of distinguishing between the effects of antioxidants other than flavonoids when found in the same foods.
Conclusion
Quercetin, and other flavonoids, have the structure to act as powerful antioxidants, and have often proven so in vitro. Quercetin, being a major constituent of the flavonoid intake, could be key in fighting several chronic degenerative diseases (Bouktaib et al. 2002). However, the degree to which quercetin is absorbed, and thus its bioavailability, leaves some doubt as to whether quercetin can exert an antioxidant effect in vivo. Further study is needed to elucidate the effects of quercetin within the body and the degree and rate of absorption, but in particular, the primary forms of quercetin found in human plasma, its metabolites, need to be used in the studies (Janisch 2004). The antioxidant activity of quercetin's metabolites and the pathways of metabolic conversion need to be identified and evaluated to accurately determine the effect quercetin has in vivo and its effectiveness in preventing diseases arising from oxidative damage.
Acknowledgments
I would like to thank Appalachian State University for providing the materials I needed to write this review. Thanks also to Dr. Claudia P. Cartaya-Marin, Dr. Lynn Siefferman, and Michael Landram for their comments and revisions.
References
Aherne, SA and N.M. O'Brien (2002) Dietary flavonols: chemistry, food content, and metabolism. Nutrition 18: 75-81.
Alexander, RB et al. (1998) Elevated levels of pro-inflammatory cytokines in the semen of patients with chronic prostatitis/chronic pelvic pain syndrome. Urology 52: 744-749.
Ansari, MA et al. (2008) Protective effect of quercetin in primary neurons against Aβ (1-42): relevance to Alzheimer's disease. Journal of Nutritional Biochemistry. Article in press.
Azuma, K et al. (2003) Enhancing effect of lipids and emulsifiers on the accumulation of quercetin metabolites in blood plasma after short-term ingestion of onions by rats. Bioscience, Biotechnology, and Biochemistry 67: 2548-2555.
Balazs, L and M. Leon (1994) Evidence of an oxidative challenge in the Alzheimer's brain. Neurochemical Research 19: 1131-1137.
Beecher, GR (2003) Overview of dietary flavonoids: nomenclature, occurrence and intake. The Journal of Nutrition 133: 3248S-3254S.
Begum, AN and J. Terao (2002) Protective effect of quercetin against cigarette tar extract-induced impairment of erythrocyte deformability. Journal of Nutritional Biochemistry 13: 265-272.
Bohm, Bruce. Introduction to Flavonoids. Amsterdam: Harwood Academic Publishers, 1998.
Boots, AW et al. (2008) In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers. Nutrition 24: 703-710.
Bouktaib, M et al. (2002) Regio- and stereoselective synthesis of the major metabolite of quercetin, quercetin-3-O-b-D-glucuronide. Tetrahedron Letters 43: 6263-6266.
Chopra, M et al. (2000) Nonalcoholic red wine extract and quercetin inhibit LDL oxidation without affecting plasma antioxidant vitamin and carotenoid concentrations. Clinical Chemistry 46: 1162-1170.
D'Abrosca, B et al. (2007) "Limoncella" apple, an Italian apple cultivar: phenolic and flavonoid contents and antioxidant activity. Food Chemistry 104: 1333-1337.
Day, AJ et al. (2001) Human metabolism of dietary flavonoids: identification of plasma metabolites of quercetin. Free Radical Res. 35: 941-952.
Graf, B et al. (2005) Flavonols, flavones, flavanones, and human health: epidemiological evidence. Journal of Medicinal Food 8: 281-290.
Kahl, R and A.G. Hildebrandt (1986) Methodology for studying antioxidant activity and mechanisms of action of antioxidants. Food and Chemical Toxicology 24: 1007-1014.
Harman, D et al. (1976) Free-radical theory of aging-inhibition of amyloidosis in mice by antioxidants-possible mechanism. American Geriatrics Society 24: 203-210.
Hollman, PCH et al. (1995) Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 62: 1276-1282.
Hollman, PCH et al. (1996) Analysis and health effects of flavonoids. Food Chemistry 57: 43-46.
Hollman, PCH et al. (1997) Bioavailability of the dietary antioxidant flavonol quercetin in man. Cancer Letters 114: 139-140.
Hollman, PCH and M.B. Katan (1997) Absorption, metabolism and health effects of dietary flavonoids in man. Biomed. & Pharmacother. 51: 305-310.
Janisch, KM et al. (2004) Properties of quercetin conjugates: modulation of LDL oxidation and binding to human serum albumin. Free Radical Research 38: 877-884.
Lotito, SB and B. Frei (2004) The increase in human plasma antioxidant capacity after apple consumption is due to the metabolic effect of fructose on urate, not apple-derived antioxidant flavonoids. Free Radical Biology & Medicine 37: 251-258.
Lotito, SB and B. Frei (2006) Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radical Biology & Medicine 41: 1727-1746.
Machlin, LJ and A. Bendich (1987) Free radical tissue damage: protective role of antioxidant nutrients. The FASEB Journal 1: 441-445.
Mariani, C et al. (2008) Flavonoid characterization and in vitro antioxidant activity of Aconitum anthora L. (Ranunculaceae). Phytochemistry 69: 1220-1226.
Mohle, B et al. (1985) UV-induced biosynthesis of quercetin 3-0-B-D-glucuronide in dill cell cultures. Phytochemistry 3: 465-467.
Moon, J et al. (2001) Identification of quercetin 3-O-b-D-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radical Biology and Medicine 30: 1274-1285.
Murota, K and J. Terao (2003) Antioxidative flavonoid quercetin: implications of its intenstinal absorption and metabolism. Archives of Biochemistry and Biophysics 417: 12-17.
Nicoteral, P and S. Orrenius (1998) The role of calcium in apoptosis. Cell Calcium 23, 173-180.
Pedersen et al. (2000) Effects of blueberry and cranberry juice consumption on the plasma antioxidant capacity of healthy female volunteers. European Journal of Clinical Nutrition 5: 405-408.
Rietveld, A and S. Wiseman (2003) Antioxidant effects of tea: evidence from human clinical trials. The Journal of Nutrition 133: 3285S 3292S.
Sakanashi, Y et al. (2008) Possible use of quercetin, an antioxidant, for protection of cells suffering from overload of intracellular Ca2+: a model experiment. Life Sciences 83: 164-169.
Scalbert, A et al. (2002) Absorption and metabolism of polyphenols in the gut and impact on health. Biomedicine & Pharmacotherapy 56: 276-282.
Shoskes, DA et al. (1999) Quercetin in Men with Category III Chronic Prostatitis: A Preliminary Prospective, Double Blinded, Placebo Controlled Trial. Urology 54: 960-963.
Uddin, S and S. Ahmad (1995) Dietary antioxidants protection against oxidative stress. Biochemical Education 23: 2-7.
US Department of Agriculture. (2003) USDA database for the flavonoid content of selected
foods. Beltsville (MD): US Department of Agriculture.
Walle, T et al. (2000). Quercetin glucosides are completely hydrolyzed in ileostomy patients before absorption. Journal of Nutrition 130: 2658-2661.
Wittig, J et al. (2001) Identification of quercetin glucuronides in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. 753: 237-243.
Young, IS and J. McEneny (2001) Lipoprotein oxidation and atherosclerosis. Biochemical Society Transactions 29: 358-362.