Author: Akash M. Shah
Institution: Arkansas State University, Arkansas Biosciences Institute
Date: February 2008
ABSTRACT
The pedunculopontine nucleus (PPN) is a mesopontine nucleus that contains cholinergic neurons and functions as part of the cholinergic arm of the reticular activating system (RAS). It is known to play a critical role in controlling the sleep-wake cycle, locomotor control, and modulation of sensory input. PPN neurons receive somatosensory and auditory input and their output is involved in coordinated flight-or-fight responses. Recent studies show that nicotine reduces the response of a subset of PPN neurons elicited by an unexpected loud sound. This dose dependent effect of nicotine on PPN output suggests that some PPN neurons express nicotinic acetylcholine receptors (nAChRs). However, the subtype of nicotine-sensitive receptors expressed by PPN neurons has not yet been identified. Therefore, this investigation was focused on identifying and localizing homomeric receptors composed of 5 α-bungarotoxin-sensitive α7 subunits, which has been indentified, most notably, in the hippocampus. Localization of α-bungarotoxin-sensitive α7 subunits containing nAChRs neurons was accomplished via labeling sagittal sections of fresh brain tissue with fluorescein labeled α-bungarotoxin (FITC α-BTX). Because α-bungarotoxin-sensitive α7 subunits were indentified in the hippocampus, its FITC labeled α-bungarotoxin labeled sagittal sections were used as the control in the project. Sections of the brainstem containing the PPN and hippocampus were stained with either nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d), FITC α-BTX or both. Stained sections were inspected with fluorescence and DIC microscopy so that stained cells could be detected. Using this technique, NADPH-sensitive neurons in PPN and FITC- α-bungarotoxin stained neurons in the hippocampus were located. However, this project was unable to locate α- bungarotoxin-sensitive α7 subunits containing neurons in the PPN. Future work to identify the subtype of nAChRs expressed by PPN neurons will rely on specific antibodies to other nAChR subtypes.
INTRODUCTION
Located in the brainstem are a group of nuclei and axon tracts collectively known as the reticular activating system (RAS). The essential parts of this system are the reticular formation (distributed through the medulla, pons and brainstem), the locus ceruleus, the raphe nuclei and the preoptic region. The RAS plays a critical role in behavioral arousal (Datta et al 1989; Curro Dossi et al 1991), regulation of muscle reflexes, and coordination of autonomic functions including breathing and heartbeat and modulation of pain sensation (Reese et al 1995a & 1995b). Recently reports show that nicotine reduces the output of reticular formation (RF) neurons in the pedunculopontine nucleus (Mamiya et al 2005; Good et al 2006). Although this strongly suggests that pedunculopontine nucleus (PPN) neurons express nicotine sensitive acetylcholine receptors (nAChRs), the type of nicotinic AChRs that mediate these responses is unknown.
The PPN has been shown to contain neurons that express muscarine-sensitive acetylcholine receptors (Olale et al 1997; Papke et al 2001). However, there has been no report of PPN neurons expressing nAChRs. Nicotinic acetylcholine receptors are ionotropic receptors activated by acetylcholine and sensitive to nicotine (Itier et al 2001; Siegel et al 1999). Several different subtypes of neuronal nAChRs have been reported. Neuronal nAChRs are composed of 5 subunits, either some combination of αx and βx subunits, or five αx subunits (Papke et al 2001). A bungarotoxin is known to be a competitive antagonist of nAChRs consisting of 5αx subunits. A BTX sensitive nAChRs have been identified in several regions of the brain, most notably, the hippocampus (Barrantes et al 1995). Because receptors composed of α7 subunits have also been reported to be very sensitive to nicotine (Barrantes et al 1995; Fu et al 1999) they were expected to be good candidates for mediators of nicotine's action in the PPN. The strong affinity of a BTX for nAChRs consisting of α7 subunits allows them to be easily identified by using α-BTX labeled with a suitable fluorophore (Barrantes et al 1995). Therefore, the purpose of this research was to determine if the nicotine sensitive cells of the pedunculopontine (PPN) neurons expressed nAChRs consisting of α7 subunits. Although sections were stained with fluorescein labeled α-bungarotoxin, no labeled cells were found in the PPN. This strongly suggests that the nicotine-sensitive cells of the PPN do not express α7 subunit containing nAChRs.
MATERIALS AND METHODS
Tissue Preparation:
Animals were used in compliance with protocols approved by the ASU IACUC and were housed in the ASU animal facility. Animals were given food and water ad libidum and were kept on a 12/12 light dark cycle. Adult male Harlan Sprague Dawley rats (300-400 g, n = 10) were used for these experiments: brains from 3 rats were used for reduced NADPH-d staining, 3 for FITC labeled α-BTX, and 4 were stained with both NADPH-d and FITC labeled α-BTX. Animals were anesthetized with ketamine and xylazine administered by intramuscular (i.m.) injection. After losing consciousness, animals were beheaded, and the brain was rapidly removed. The brain was immediately placed in cool (~0oC) artificial cerebral spinal fluid (ACSF, pH 7, 122.8mM NaCl, 5.0 mM KCl, 1.2 mM MgSO4, 2.5 mM CaCl2, 1.2 mM HaH2PO4, 25 mM NaHCO3, 10 mM Glucose). After cooling for 3 to 5 minutes each brain was bisected along the mid-sagittal fissure and the brain stem was removed for sectioning.
Sectioning:
The brainstem was attached to a metal stage with super glue with the cut (medial) side up. Sections were cut using a Vibratome® (Model 1500) microtome. Because the tissue was unfixed, the sectioning tray was surrounded by crushed ice so that the tissue temperature was maintained at ~0oC. Section thickness was ~140 µM. As soon as a section was cut it was placed in cold ACSF.
Because the PPN lies between 1.6 and 2.1 mm lateral to the midline of brainstem (Paxinos and Watson 1988) about 10 to 15, sections from each brain hemisphere were cut and stained. After sectioning tissues were fixed in 4% formaldehyde (from paraformaldehyde) for ~15 minutes. Fixed section tissues were stained with either FITC α-BTX or NADPH-d.
Staining:
Each section was stained using one of three different techniques; NADPH-d, FITC α-BTX, or both. In order to compare results of FITC α-BTX and NADPH-d staining, every other sections of a serial sequence were stained separately in either FITC α-BTX or NADPH-d.
NADPH-d was used to stain nitric oxide (NOS)-expressing neurons. Although this technique is routinely used to stain cholinergic brainstem neurons (Bredit et al 1991; Hope et al 1991), it does not differentiate between cells expressing different subtypes of nAChRs. On the other hand, FITC α-BTX binds to cells expressing nAChRs consisting of α7 subunits, but not to those that express other AChRs.
NADPH-d staining:
After fixation sections were incubated in NADPH-d solution (ph 8.0, Malic acid 15 mM, NADP 1.0 nM, Nitro blue tretrazolium 0.2 mM, TRIS and 0.3% Tween 20) for 1 hr at 37◦C. Sections were then rinsed in 0.1 M phosphate buffered saline (PBS) for 5 min. After staining each section was transferred onto a gelatin coated slide. Slides were covered and air dried for 24 hours. After drying sections were dehydrated in graded (70%, 95%, 100%) ethanol solutions followed by xylene. Covers slips were mounted with non-fluorescent mounting medium Fluoromount-G (Electron Microscopy Science, Washington, PA). Sections were inspected using a Nikon Eclipse 800 epifluorescence microscope.
FITC α-BTX labeling:
After fixing sections were incubated for 2 hrs in 100 µg/ml of FITC α-BTX (Sigma-Aldrich). After staining sections were placed in 4% formaldehyde for 30 min, rinsed in 0.1 M PBS, mounted and inspected as described above.
Microscopy:
Sections were inspected using a Nikon Eclipse E800 epifluorescence microscope equipped with an Hg-vapor lamp and a Cascade Photometric camera controlled by Meta-Morph software. FITC-α-BTX sections were examined and imaged using a FITC filter cube (excitation: 490nm, emission: 520nm).
RESULTS
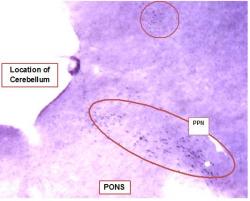
In NADPH-d stained sections, cholinergic brainstem neurons were stained dark blue because of an insoluble precipitate from the nitroblue tetrazolium chromogen and are clearly visible. In sections cut between 1.5 and 2.0 mm lateral of the midline, the PPN was easily identified by its location, the presence of large cholinergic neurons and its characteristic shape. A portion of a representative NADPH-d stained section containing the PPN and other portions of the RF is shown in Figure 1. In these sections hippocampal neurons were not labeled, but the hippocampus could be easily identified by its characteristic shape.
In sections cut from the same region and stained with FITC α-BTX hippocampal neurons were brightly fluorescent. An image of a portion of the hippocampus contained in one of these sections is shown in Figure 2. However, in the sections stained with FITC α-BTX no fluorescent cells in the brainstem could be found even though hippocampal cells in the same section were brightly labeled. Hippocampal pyramidal neurons are known to express α-bungarotoxin-sensitive α7 subunits and be labeled with α-BTX (for review see Palacios and Mengod, 1989) this allowed them to be used as positive controls for FITC α-BTX labeling, after comparing FITC α-BTX labeled hippocampus images to FITC α-BTX labeled PPN images, I reached a conclusion that PPN does not contain α-bungarotoxin-sensitive α7 subunits.
Figure-1: Low Magnification Image of Pedunculopontine Nucleus Neurons Labeled with NADPH-diaphorase. Image of a portion of a ~140µM thick representative section of fresh (unfixed) brain cut ~1.8 mm lateral of the midline. Section was stained with nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d). The dark purple staining is produced by an insoluble precipitate from the nitroblue tetrazolium chromogen and is specific to cholinergic neurons. Cholinergic PPN neurons are labeled and easily identified by their dark staining, large size and location. Other stained neurons are visible throughout the upper portion of the image (circled). Image was made using a dissection scope, approximate magnification 10X. Cerebellum was sectioned and stained, but is not shown in this image.
Figure-2: Black and White Epifluorescence Image of labeled Pyramidal Hippocampal Neurons. Image of a portion of a ~140µM thick representative section of fresh (unfixed) brain cut ~1.7 mm lateral of the midline. Section was stained with fluorescein α bungarotoxin (FITC α BTX). BTX is a competitive antagonist of α7 subunits of nicotinic acetycholine receptors (nAChR) and is used to label cells expressing this form of the nAChR. In this image, pyramidal neurons are brightly fluorescent and easily identified. Image was made using a Nikon Eclipse E800 epifluorescence microscope (excitation: 490nm, emission: 520nm) equipped with an Hg-vapor lamp and a B&W Cascade Photometric camera. No labeled brainstem cells were observed in this section. Magnifications 1000X.
DISCUSSION AND CONCLUSIONS
The goal of this investigation was to use histochemical methods to determine the type of nAChRs in the reticular activating system. Although many techniques can be used to investigate receptor expression, this method was selected because these techniques label intact surface receptors, suggesting that labeled receptors are functional. Determining the type of functional nAChRs expressed by PPN neurons was of interest because we had recently discovered that a subset of RF neurons (in the PPN) exhibit a dose-dependent response to acute exposure to nicotine (Mamiya et al 2005) or cigarette smoke (McKeon et al, 2005). While this strongly suggests that these neurons express functional nAChRs, the type of nAChRs is not known. Identifying the specific type of nAChRs expressed by these neurons is important to understanding the response of these neurons and how it might be affected by chronic exposure to nicotine and cigarette smoke. This knowledge has obvious health implications, and is also important for future investigations that would utilize specific antagonists and agonists to modify nAChR-mediated responses.
Although sectioning unfixed brain tissue presents a challenge, the use of unfixed material allowed standard fluorescence microscopy techniques to be used to identify cells expressing α-bungarotoxin-sensitive receptors. Using unfixed tissue avoided interference from the autofluoresence induced by fixation and allowed a clear signal from the FITC α-BTX labeling to be detected.
We attempted to identify and localize homomeric receptors composed of 5 α-bungarotoxin-sensitive α7 subunits in unfixed brain sections by labeling them with FITC α-BTX. This type of nAChRs has been found in other regions of the brain, most notably, in the hippocampus (Barrantes et al 1995). While we were able to label hippocampal neurons using this technique, no α-bungarotoxin labeling of RAS neurons was found. NADPH-d has been shown to be a selective marker for nitric oxide-synthase (Bredit et al 1991; Hope et al 1991). Because nitric oxide-synthase is localized to cholinergic cells in the brainstem of adult rats, it has been used to detect cholinergic brainstem neurons including those in the PPN (Skinner et al 1989; Bredit et al 1991; Lanca et al 2000). Using this technique we were able to identify cholinergic neurons in the PPN and other brainstem nuclei. Although NADPH-d is useful for labeling cholinergic cells, it cannot be used to differentiate cholinergic neurons by the specific subtypes of nAChRs they express. On the other hand, α bungarotoxin is a highly selective competitive antagonist of the α7 subunit of nAChRs and is used to identify cholinergic cells that express this nAChR subunit on their surface (Barrantes et al 1995).
Hippocampal pyramidal neurons express high levels of α7 subunit containing nAChRs (Barrantes et al 1995). Therefore to verify efficacy of FITC α-BTX staining protocols, alternate serial sections that contained the hippocampus and the RF were stained with FITC α-BTX. In these sections, hippocampal pyramidal cells were brightly labeled (Figure 2), but no labeled cells were visible in the RF. However when sections from the same series were stained with NADPH-d, labeled cells were observed in the PPN (Figure 1). This result suggests that the nicotine-sensitive cholinergic cells of the PPN do not express functional nAChRs containing α7 subunits. This is new information that allows the effort to identify the subtypes of nAChRs expressed by nicotine-sensitive PPN neurons to be refined.
The results from NADPH-d staining agree with previous reports that the PPN contains cholinergic cells. However, the absence of fluorescein labeled cells in the PPN showed that nAChRs containing α-bungarotoxin-sensitive α7 subunits of are not expressed on the surface of PPN cholinergic neurons. Future work to identify the subtype of nAChRs expressed by PPN neurons will rely on specific antibodies to other nAChR subtypes and electrophysiological techniques (Good et al 2006) to further describe the nicotinic pathway in the RF and how it is modulated by nicotine and cigarette smoke.
REFERENCES
Barrantes GE, Rogers AT, Lindstrom J and Wonnacott S (1995) -Bungarotoxin binding sites in hippocampal and cortical cultures: Initial characterization, colocalization with 7 subunits and up-regulation by chronic nicotine treatment. Brain Res 672: 228-236.
Bredit, DS, Glatt, CE, Hwang, PM, Foutuhi, M, Dawson, TM, Snyder, SH, 1991. Nitric oxide synthase protein mRNA is discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron 7:615-624.
Curro Dossi R, Paré D, Steriade M, 1991. Short-lasting nicotinic and long-lasting muscarinic depolarizing responses of thalamocortical neurons to stimulation of mesopontine cholinergic nuclei. J Neurophysiol 65:393-406
Datta, S, Paré D, Oakson G and Steriade M, 1989. Thalamic-projecting neurons in brain stem cholinergic nuclei increase their firing rates one minute in advance of EEG desynchronization associated with REM sleep. Neuroscience Abstracts, 15:452.
Fu Y, Matta SG and Sharp BM, 1999. Local α-Bungarotoxin-Sensitive Nicotinic Receptors Modulate Hippocampal Norepinephrine Release by Systemic Nicotine. The Journal of Pharmacology and Experimental Therapeutics, 289(1):133-139.
Good CH, Bay KD, Buchanan RA, McKeon KA, Skinner RD and Garcia-Rill E, 2006. Prenatal exposure to cigarette smoke affects the physiology of pedunculopontine neurons in development. Neurotoxicology and Teratology, 28(2):210-9.
Hope BT, Maicael GJ, Knigge KM, Vincent SR, 1991. Neuronal NADPH diaphorase is a nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America 88:2811-2814.
Itier V and Bertrand D, 2001. Neuronal nicotinic receptors: from protein structure to function. Edited by Andreas Engel and Giorgio Semenza. FEBS Letters, 504(3):118-125.
Lanca, AJ, Adamson, KL, Coen KM, Chow, BLC, Corrigall WA, 2000. The pedunculopontine tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: a correlative neuroanatomical and behavioral study. Neuroscience 96:735-742.
Mamiya N, RA Buchanan, RD Skinner and E Garcia-Rill, 2005. Nicotine suppresses the P13 auditory evoked potential by acting on the pedunculopontine nucleus in the rat. Exp Brain Res, 164(1):109-119.
McKeon K, R. Skinner, E Garcia-Rill, R Buchanan, 2005. Effects of cigarette smoke on P13 potential amplitude and habituation in rats. Neurosci Abst 31:1028.15
Olale F, Gerzanich V, Kuryatov A, Wang F and Lindstrom J, 1997. Chronic nicotine exposure differentially affects the function of human alpha3, alpha4 and alpha7 neuronal nicotinic receptor subtypes. Journal of Pharmacology and Experimental Therapeutics, 283:675-683.
Palacios, JM and G Mengod, 1989. Radiohistochemistry of receptors in the hippocampus: Focus on the cholinergic receptors. In Neurology and Neurobiology Vol 52: The Hippocampus. New Vistas. Eds V Chan-Palay and C Köhler. Alan R. Liss, Inc., New York, NY. Pp 207-224.
Papke RL, Sanberg PR, and Shytle RD, 2001. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. The Journal of Pharmacology and Experimental Therapeutics, 297(2):646-656.
Paxinos G and Watson C, 1998. The Rat Brain in Stereotaxic Coordinates, 4th edition. The Academic Press, 525 B Street, Suite 1900, San Diego, California 92101, USA, Figure 81-84, Plates 81-84.
Reese NB, Garcia-Rill E and Skinner RD, 1995a. Auditory input to the pedunculopontine nucleus. II. Unit Responses. Brain Research Bulletin, 37:265-273.
Reese NB, Garcia-Rill E and Skinner RD, 1995b. The pedunculopontine nucleus,auditory input, arousal and pathophysiology. Progress in Neurobiology, Vol 42. pp105-133. Elsevier Science LTD. London.
Siegel GJ, Agranoff BW, Fisher SK, Albers RW, and Uhler MD, 1999. Basic Neurochemistry: Molecular, Cellular and Medical Aspects, Sixth Edition. GABA Receptor Physiology and Pharmacology. American Society for Neurochemistry. Lippincott Williams and Wilkins.
Skinner RD, Conrad N, Henderson V, Gilmore S, Garcia-Rill E (1989). Development of NADPH diaphorase positive pedunculopontine neurons. Exp Neurol 104:15-21.