Date: August 2007
This is a guest article contributed to JYI and we are immensely thankful to the authors:
Kiersten Israel-Ballard, MPH (doctoral candidate, School of Public Health, University of California, Berkeley) and Caroline Chantry, MD (Associate Professor of Clinical Pediatrics, University of California Davis Medical Center)
Imagine you are Rebecca. Or that she is your sister, your aunt, or your friend. Rebecca lives in a semi-urban town in South Africa - an illegal settlement made up of homemade shacks with limited access to clean water and toilets. At the age of 24, Rebecca is happy to be having a baby, since her partner wanted to start a family. She doesn't live with him yet, though he tells her he is saving to get a place for the two of them. He has been telling her this for 3 years. For now, Rebecca lives with her sister, who has her own shack that she shares with her daughter.
Rebecca learned that she was HIV positive during a routine HIV test at her antenatal check-up. Because she is afraid that her sister will be angry and her partner will leave her, she does not share her secret of being HIV infected with anyone. At the clinic where she had her check-up, a counselor talks to Rebecca about exclusive breastfeeding. Rebecca did not realize that she could breastfeed even though she was infected. The counselor explains that although breast milk sometimes carries the HIV virus, it protects the baby from many other dangerous diseases and is still one of the safest ways to feed the baby. It's a better option than formula, the counselor explains. Without access to clean water, Rebecca couldn't be sure the formula she mixed was safe. And if she ran out of money for buying formula, her baby would go hungry. Even the free formula at the clinic would take time to get and would not always be available.
The counselor promises to help Rebecca with the breastfeeding once she has delivered her baby. She is happy to breastfeed because then nobody will think she is infected. But once Rebecca delivers, she is told by the nurses at the hospital that she should not breastfeed her baby because she can infect him. This scares and confuses her.
Rebecca finally decides to tell her partner that she is HIV positive. He is angry but tells her he wants her to use formula, and that he will buy it. She gives up on breastfeeding and uses the formula. Her partner buys her one can and tells her he will buy more when she needs it. He does not buy more. When the can of formula runs low, she adds water to make it last longer. When it is finally finished, she gives the baby sugar and water. Since she didn't breastfeed, her breast milk has dried up and there is nothing to feed her baby.
Rebecca knows her baby is not well because he has had diarrhea now for over 2 weeks. When she takes the baby to the clinic, they tell her the baby is dehydrated and in serious condition. Looking at her tiny baby, she is very afraid because she has already lost one child. She wonders if she did something wrong and is desperate to save her baby.
Millions of HIV positive women in developing countries face the desperate dilemma- breastfeed their babies and risk HIV transmission or give infant formula and risk illness and possible death from diarrhea and other diseases
Rebecca is one of 13.5 million women living with HIV in sub-Saharan Africa, the majority of who are of child-bearing age. In South Africa in 2005, one in three women aged 30-34 were living with HIV1. Such mothers face a desperate dilemma breastfeed their baby and risk HIV transmission or give infant formula and risk illness and possible death from diarrhea.
The issues surrounding infant feeding for those born to HIV positive women in developing countries are complex and have been the focus of much debate among HIV researchers and nutritionists. It has yet to be resolved. How do you remove breast milk in Africa, a pillar of infant survival, for the prevention of only one disease when so many other health issues are also at stake? These challenges prompted our research team to investigate a long-ignored potential method for infant feeding heat treatment of breast milk. In order to fully appreciate why such an unusual option may hold promise, we present below a review of peri-natal HIV transmission and the challenges of infant feeding.
Mother-to-Child Transmission of HIV
Mother-to-child transmission (MTCT) is responsible for approximately 90% of the 725,000 HIV infections that occur each year among the world's children; of these 90% are in sub-Saharan Africa2. In developed countries a dramatic reduction in mother-to-child transmission of human immunodeficiency virus (HIV) has been achieved due to antiretroviral drugs, elective Caesarean section, and avoidance of breastfeeding. However, these prevention strategies may not be available to women and infants in developing countries such as in sub-Saharan Africa, where rates of HIV prevalence among pregnant women have soared as high as 15-43% in some regions2.
Peri-natal HIV transmission can occur in utero, intrapartum, or post-partum during breastfeeding. It is estimated that 200,000 to 350,000 infants contract HIV via prolonged breastfeeding each year2,3. Yet in resource-poor areas lacking in appropriate and nutritionally adequate breast milk substitutes and supplemental foods, breastfeeding for extended periods is widespread. Even the low cost two-dose nevirapine prophylaxis does not substantially decrease transmission from prolonged breastfeeding3-5.
Mechanism of Breastfeeding Transmission
The mechanism by which HIV is transmitted through breastfeeding is only partially understood. Maternal CD4+ count, nipple pathology, breast milk viral load, mastitis, and duration and exclusivity of breastfeeding are important factors in determining associated risk of HIV transmission6. In the absence of antiretroviral medication, the estimated risk of a neonate born to an HIV-infected mother acquiring HIV peri-natally, ranges from 15-30% if not breastfed, 25-35% if breastfed through 6 months, and 30-45% if breastfed 18-to-24 months7. HIV occurs in breast milk in cell-free and cell-associated form; recent data suggest that cell-associated HIV has a more important role in transmission of HIV via breast-feeding than does cell-free virus8.
Additionally, breastfeeding patterns may be critical, in that exclusive breastfeeding (EBF) may be protective against HIV transmission compared to partial or mixed breastfeeding. An observational study in South Africa found that the cumulative probability of HIV infection by 6 months of age was similar among the EBF (0.194 [95% CI, 0.125-0.274]) and the never breastfed (0.194 [95% CI, 0.136-0.260]) groups, but was highest among those who were mixed feeders (0.261 [95% CI, 0.205-0.319]) 9. This increased risk among mixed feeders is hypothesized to be due to the introduction of antigens and bacterial contaminants in food and water and the increased risk of mastitis in the mother10,11. This finding has been confirmed in several recent studies. The Zvitambo study group in Zimbabwe found that mixed feeding doubled transmission risk at 18 months compared to EBF. A study in Cote d'Ivoire found that mixed feeding increased the risk of transmission five-fold compared to EBF12,13. A recent meta-analysis by the Breastfeeding and HIV International Transmission Study Group found that postnatal transmission may be responsible for up to 42% of MTCT14. This risk was constant over 24 months with the risk from breastfeeding for 6 months at only 4%, emphasizing that longer durations of breastfeeding are associated with increased transmission risk.
Antiretroviral Therapy and Breastfeeding Transmission
Increased efforts are underway in developing countries to provide short-course antiretroviral therapy (ART) peri-natal prophylaxis to reduce MTCT. The duration of effective prophylaxis during breastfeeding provided by different regimens is unclear. Notably, the Ivory Coast trial with zidovudine15 and the HIVNET 012 trial with nevirapine4 demonstrated that significant prophylactic efficacy was maintained in breastfeeding infants through 3 and 4 months respectively. Recent studies have shown that prophylactic treatment of the mother antepartum and intrapartum and of the infant during breastfeeding results in low cumulative rates of transmission at 6 months (5.1%)16,17. Some regimens are focusing on reducing transmission that occurs through early breastfeeding. For example, HIVNET 02318, is investigating extended regimens of nevirapine dosing in an attempt to reduce additional breastfeeding transmission6. Most recent data presented at the 2007 International AIDS Society Conference on HIV Treatment and Pathogenesis, showed that in one study in Tanzania, ART and breastfeeding was associated with a cumulative transmission at 6 months of only 5.0% (less than 1% due to breastfeeding) 19 and in another study in Rwanda, only one out of 174 (0.6%) breastfeeding women on ART transmitted HIV to her infant20. Single-dose intrapartum nevirapine reduces intrapartum HIV transmission, however it selects for nonnucleoside reverse-transcriptase inhibitor resistance in breast milk and plasma21,22. It is unclear if short-course prophylaxis provides a more prolonged effect, however, it is known that breast milk viral loads are associated with plasma viral loads23. This correlation notwithstanding, some HIV-infected women receiving combination ART and with low or undetectable plasma viral loads have demonstrable HIV provirus in their breast milk24. Nevertheless, ART that lowers maternal viral load may cause lower breast milk titers, and the single intrapartum nevirapine dose may decrease maternal plasma viral loads by 10- to 1000-fold. ART may also have an independent effect on breast milk viral load. The global ART roll out goal is to provide nevirapine during labor and delivery and ART throughout the lifetime of both mother and infant as needed. Currently, however, only 20% of those who need ART have access to it25.
Risks of Formula Feeding
Although formula is the recommended infant feeding option for HIV positive mothers in developed countries26, for many reasons this may not be a feasible or preferred choice for women in resource-poor communities. First, for many families the cost of formula may be prohibitive and more affordable local foods, (e.g. WHO-recommended replacement foods for southern Africa, such as full cream milk), do not meet micronutrient and fatty acid requirements for infants less than 6 months, even with the addition of available micronutrient supplements27.
Second, even with sufficient financial resources some settings lack the infrastructure to ensure consistent and safe formula availability during political crises or perilous weather conditions28.
Third, social attitudes and cultural beliefs about breastfeeding may be so strong that even when educated about the risk of HIV transmission, many women in Africa still choose to breastfeed, and those who do not may face isolation and social stigma29. Nduati et al. showed that even when safe water to prepare formula was available, only 70% of women randomized to formula feed their infants actually did so exclusively30.
A fourth issue relates to the concern that provision or endorsement of formula for infants of HIV-infected mothers may have a significant spill-over effect to the normal population, further increasing infant mortality in these regions31.
Fifth, the reduced duration of lactational amenorrhea and subsequent increase in estimated births in some countries attributable to not breastfeeding may ultimately result in an increase in the number of HIV-exposed and infected infants31-33.
Finally, formula-fed infants have a substantially increased risk of mortality due to non-HIV infectious diseases in developing countries, where unsanitary water conditions and escalating HIV rates exist34. In contrast to the estimated 200,000-350,000 infants who contracted HIV through breastfeeding in 200035,WHO estimates that 1.5 million infants died because they were not optimally breastfed36. In one study, formula feeding was associated with a 14-fold increase in diarrhea-associated mortality for all infants and a 25-fold increased risk in infants less than 2 months old37. A recent study in Ghana, India and Peru found that non-breastfed infants had a 10-fold higher risk of dying when compared to predominantly breastfed infants38.
Accordingly, it is important that the overall goal be to improve infant survival, not just reduce transmission of HIV. This is illustrated by a randomized trial of infant feeding among HIV positive mothers in Kenya39,40. Formula use significantly increased HIV-free infant survival over a 2-year period, but cumulative child death rates were not significantly different between formula and breast-fed infants (20.0% versus 24.4%, p value of less than 0.3). Despite access to clean water and free formula, more than half of the deaths in the formula-fed group occurred during the first 6 months of life, compared to 36% of the deaths in the breastfeeding group.
Thus, for all of the above reasons, it is becoming increasingly and painfully clear that infant formula is not a global answer to improving survival of children born to HIV positive mothers in resource-poor areas. The severe implications of formula feeding when adequate infrastructure is not in place were recently demonstrated in Botswana. The National PMTCT PEPFAR-funded program distributed free infant formula to all HIV positive mothers. Between January and March 2006 there were 23,998 cases of diarrhea and 486 deaths due to water contamination and feeding infant formula, a 4-fold increase in diarrhea cases and 25-fold increase in diarrhea deaths in children less than 5 compared with 2004 to 2005. Those most affected were children 0-12 months old, and the primary risk factor for diarrhea (adjusted OR=50.0) and death (OR=8.5) was not breastfeeding41. A study of 154 inpatients with diarrhea, 90% of whom were not breastfed, found that none of the breastfeeding children died, compared with 23% of the non-breastfeeding children42. In addition, the infants who died had received an average of 51% of the PMTCT program infant formula they should have received after birth, resulting in growth faltering43. Severe acute malnutrition following diarrhea, not HIV status, was the most important predictor of death and correlated with lack of sufficient formula, These findings prompted the Centers for Disease Control and Prevention to recommend that the formula policy needs review, given that data show women who are exclusively breastfeeding, have high CD4+ levels or are on ART, have lower risk of HIV transmission. Additionally, safety cannot "be assumed" and adequate amounts of formula and safe water must be ensured.
This combination of cost, sustainability, safety, health, child spacing, and socio-cultural factors, such as acceptability, may explain why formula feeding as an option for HIV-infected mothers in developing countries has been met with considerable rejection and skepticism28,31,44,45.
Benefits of Breast milk
The important immunological and anti-infective factors naturally found in human milk have been well-documented46,47. Examples of important vertically transferred anti-infective components include proteins such as secretory IgA, lactoferrin, lysozyme, haptocorrin, and lactoperoxidase, all of which protect against enteric and other pathogens. This biochemical protection manifests itself in lower morbidity and mortality rates among exclusively breastfed infants. EBF also promotes gut maturation48 (which may explain the lower risk of HIV transmission9,12,13,49); propagates the development of beneficial microflora that lower intestinal pH and prevent the growth of pathogens50; and promotes successful lactogenesis which reduces breast inflammation and may also decrease HIV transmission11. Simulated estimates of adverse outcomes in impoverished developing countries have shown that the risk of mortality due to infectious diseases among non-breastfed infants would surpass the risk of HIV transmission among breastfed infants, especially in infants less than 6 months of age51-53.
Recommended Infant Feeding Options for HIV Positive Mothers
In light of the overall protection offered through breastfeeding, a statement issued in 1998 by WHO, UNAIDS, and UNICEF stated that "breastfeeding should continue to be protected, promoted, and supported" and women should be counselled regarding "the risks and possible advantages associated with other methods of infant feeding26." Current WHO recommendations state that when replacement-feeding options are acceptable, feasible, affordable, safe, and sustainable (AFASS), avoidance of all breastfeeding is recommended; otherwise exclusive breastfeeding is recommended for the first six months of life followed by weaning only if a nutritionally adequate and safe diet is maintained54-56."Current WHO recommendations state that when replacement-feeding options are acceptable, feasible, affordable, safe, and sustainable (AFASS), avoidance of all breastfeeding is recommended; otherwise exclusive breastfeeding is recommended for the first six months of life followed by weaning only if a nutritionally adequate and safe diet is maintained."
Alternatives to exclusive breastfeeding include: breast milk substitutes (commercial infant formula, home-prepared infant formula); modified breastfeeding (early cessation, expressed and heat-treated breast milk); and other (breast milk banks, wet nursing) 26. Support for and promotion of low-cost, AFASS feeding alternatives that meet the WHO criteria are desperately needed for HIV-infected mothers, the majority of whom live in resource-poor areas of sub-Saharan Africa57. In October 2006, when WHO modified their recommendations to note that breastfeeding should continue until replacement feedings are AFASS, they also called for governments and donors to increase commitment and resources to implement the UN HIV and Infant Feeding Priority Actions. These five priority actions include conducting country level assessments of the acceptability, affordability, feasibility, sustainability and safety of the different infant feeding options in order to prevent postnatal HIV transmission and improve HIV-free child survival.
Challenges of Early Cessation of Breastfeeding: Diarrhea and Malnutrition
Recent data suggest that the weaning period after months of EBF is one of high-risk for the infant. This risk is two-fold: the absence of breast milk's immunological and nutritional benefits and concomitant introduction of contaminants into the diet result in increased susceptibility to diarrhea morbidities and lack of adequate nutrition. Although early cessation of breastfeeding reduces HIV transmission, morbidity and mortality has been shown to actually increase58. Indeed, this was a major concern emphasized during the latest XVI International AIDS Conference in Toronto in August 2006 and XIV Conference on Retroviruses and Opportunistic Infections in Los Angeles in February 2007.
Piwoz, et al. and DePaoli et al. reported that practical difficulties existed for mothers and they required careful preparation and support regarding breast care, lactation suppression, and coping to be successful with rapid cessation59,60. Johnson et al. found that available foods in Mozambique did not meet the infant's nutritional needs following EBF, increasing the risk of malnutrition61. Becquet et al. recently found that inadequate complementary feeding of infants at 6 months in Cote d'Ivoire was associated with a 37% increased probability of stunting62. Kourtis et al. and the Breastfeeding Antiretrovirals Nutrition (BAN) study reported that among infants who had been exclusively breastfed, an increase in diarrhea was observed around the time of weaning that continued through the first year63. Kafulafula et al. found that gastroenteritis frequency among infants receiving ART prophylaxis in Malawi was highest immediately following weaning64. A Zambia Exclusive Breastfeeding Study (ZEBS) randomized trial led by Sinkala et al., showed no significant difference in HIV-free survival at 24 months between infants who underwent abrupt cessation of breastfeeding at 4 months and infants who continued breastfeeding for the duration of the mother's informed choice65. Thomas et al. with the Kisumu Breastfeeding Study in Kenya found that increased risk of diarrhea and hospitalizations were associated with weaning after EBF66. Onyango et al. found that infants enrolled in a peri-natal prevention trial in Uganda had increased risk of serious gastroenteritis and death after breastfeeding cessation67. High rates of diarrhea and mortality of infants associated with early cessation of breastfeeding (at or before 6 months of age), presumably are due in part to the sudden lack of immune protection in complementary foods that had been available during EBF. Moreover, a recent study in Zambia found a significant increase in breast milk viral load during the weaning period (p=0.0001), suggesting that if a mother were to breastfeed during this time, the risk of HIV transmission to the infant could be substantially increased68.
These data illustrate that focusing only on EBF for the first months is not enough to ensure positive health outcomes for these infants. Safe and nutritious infant feeding options are clearly needed for infants as they transition from EBF to replacement feeds or add complementary foods.
Heat-treated Breast milk: A Possible Compromise
WHO, UNICEF, and UNAIDS recommend manually expressed, heat-treated breast milk as one alternative to breastfeeding for HIV positive mothers in developing countries. To date, its implementation has not been adequately addressed, researched, supported and/or promoted31,69-75.
WHO endorsed heat treatment methods include:
1) Pasteurization of breast milk. While exact methods are not described, commercial pasteurization of human milk is typically done by heating it to 62.5°C for 30 min, a method known as Holder pasteurization that retains the majority of breast milk's protective elements. This method, widely used in human milk-banking, has been reported to inactivate both cell-free and cell-associated HIV76-79, although this has not been tested in undiluted whole human milk76,78,79. Milk is a complex medium and has been shown to exhibit thermal protection thus influencing heat inactivation of organisms80,81. However, the need for temperature gauges and timing devices limits practicality of Holder pasteurization in most at-risk communities82.
2) Boiling by placing the milk in a pan with no water bath (time unspecified) has been recommended in WHO training manuals83. This method most likely causes significant nutritional damage. Yet, to our knowledge, it has never been fully evaluated scientifically. An additional method recently proposed, Pretoria pasteurization84, involves boiling a pan of water, removing it from the heat source, and immediately placing a covered glass peanut butter-type jar of breast milk in the water for 20 min85. Jeffery, et al. investigated the ability of Pretoria pasteurization to denature HIV. Although they showed no evidence of HIV replication in Pretoria pasteurized samples using co-culture techniques, the significance of these results may be compromised since they were unable to consistently detect HIV in the unheated controls as well. Moreover, our recent pilot study showed residual cell-free HIV as detected by the reverse transcriptase assay remaining in Pretoria pasteurized samples.
The lack of data verifying the nutrient content of boiled breast milk and the impractical protocol for implementing Holder Pasteurization, combined with the skepticism surrounding acceptability of manually expressing and heating breast milk, may be reasons why many health care professionals hesitate to promote the WHO recommendations as a feasible option to mothers who are in need of realistic alternatives. Our multidisciplinary team of researchers sought to address this gap in knowledge. We designed Flash-heat, a simple passive heat transfer technique that a mother can easily employ in her kitchen or over a fire, and then investigated its acceptability and safety.
Potential role for Flash-heated breast milk
The data presented above illustrate the risks encountered during early and rapid cessation of EBF. Inadequate feeding practices due to lack of appropriate replacement feeds often result in malnutrition and growth faltering. As well, an increase in breast milk viral load or mixed feeds may increase the risk of transmission. Perhaps heat-treated breast milk could be used during these high-risk times and could be viewed as a replacement food; it is HIV-free, nutritious, affordable and available. Milk production would be well established after months of EBF, and other complementary foods would then be an additional source of infant nutrition. If a mother fed heat-treated breast milk to her infant several times each day while introducing complementary foods it could significantly impact the infant's nutritional intake as well as provide immune protection unique to breast milk, thus lowering risk of morbidity from non-HIV diseases. While some mothers may provide heated milk only for weeks and others for months, the contribution to the infant's health outcomes during this transition phase could still be substantial.
Demonstrating the acceptability and safety of Flash-heat
In 2001, we conducted a qualitative study in Zimbabwe to assess attitudes of heat-treating breast milk86. The 77 respondents (mothers, fathers, grandmothers, and birth attendants), in 13 focus groups were initially skeptical, but education that the method could destroy HIV and further discussion led the majority of participants to conclude that if nutritious and safe, heated breast milk could be a viable, sustainable, and low cost alternative to formula in their communities. Additionally, we worked with the University Research Co, LLC (URC) in Tanzania in July 2006 to further address acceptability and potential feasibility of heat-treating breast milk and to obtain preliminary data for this proposed feasibility study (unpublished data). Thirty-two in-depth interviews were conducted with health care workers and HIV positive mothers in urban and rural areas. Many of the views expressed were similar to those in Zimbabwe but we also found that demonstrating Flash-heat (vs. just explaining it) during counseling is critical, and that the primary barrier to comprehensive counseling was the severe staffing shortage. Thus, these qualitative studies suggested that some women are willing to attempt to heat-treat their milk to prevent MTCT of HIV to their infant. As summarized by one nurse midwife: "But they would benefit because even a poor mother who can't afford these artificial feeds, they could do it because she's got the breast milk."
The Flash-heat method involves manually expressing 50mL of breast milk into an uncovered glass peanut butter jar, which is then placed in 450mL of water in an aluminum pan. The water and milk are heated together over a high flame until the water first reaches a rolling boil. The breast milk is immediately removed from the water, covered, and allowed to cool and then cup or spoon-fed to the infant. Field observations using local stoves, utensils, pans, and jars and varying water and milk volumes in South Africa, Kenya and Tanzania have shown that this protocol can be adapted as long as the water level is approximately two finger-widths above the milk level. Flash-heat is a quick method, typically reaching temperatures above 56.0C for over 6 min and peaking around 73°C. This is similar to the high temperature, short time (HTST) heat treatment method (72°C for 15 sec) preferred for commercial milk pasteurization, because it effectively kills bacteria and cytomegalovirus, with no decrease in vitamins, lactoferrin, total IgA concentrations or secretory IgA activity87-90.
Figure 1: A community health worker uses simple, locally purchased materials to demonstrate a method of flash-heating milk in Kibera, a massive slum area outside of Nairobi, Kenya. Although the demonstration included temperature gauges, the technique can also be implemented without thermometers
Figure 2: Materials used for the demonstration included cow's milk, a locally purchased glass jar, a locally purchased aluminum pan, and a small charcoal stove that is commonly used for cooking. The temperature probes used in the test are not required in actual practice
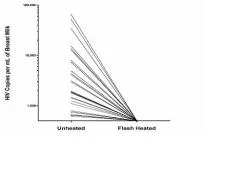
Our pilot data show that Flash-heat was capable of inactivating "spiked" cell-free HIV-1 as detected by reverse transcriptase (RT) activity91. We have also recently confirmed that Flash-heat inactivated cell-free HIV in 30 naturally infected breast milk samples collected from HIV positive mothers (Figure 2) 92. Preliminary data also suggests inactivation of cell-associated HIV.
Our pilot data also suggest limited impact from Flash-heat on vitamins and proteins91. To confirm these findings, we utilized 50 of the milk samples collected from HIV positive mothers in South Africa, as described above, for vitamin (vitamins A and B12, ascorbic acid, riboflavin, pyridoxal, and folate) assays. Significant differences in some vitamin concentrations were observed between Flash-heated and unheated breast milk, several due to an increase in concentration detected in post-heat samples hypothesized to be caused by release of vitamins from binding proteins in the milk. The percent remaining after Flash-heat suggests that most vitamin concentrations are retained post-heat and that this could be a practical and nutritious infant feeding method for mothers in developing countries who need safe alternatives for infant feeding93.
Figure 3: Log scale comparison of copies/mL of cell-free HIV-1 as detected by RT activity in unheated versus Flash-heated naturally infected breast milk samples (n=30)
Samples were also assayed for immunoglobulin (Ig) levels and bioactivity. Although Flash-heat did result in significant decreases in total and some antigen-specific secretory IgA and IgG concentrations, most impacted was poliovirus IgA, of which 2/3 remained intact post-heat94. Measurable influenza virus IgA and IgG concentrations increased. This suggests that the majority of breast milk antibodies survive Flash-heat, confirming that Flash-heated breast milk is immunologically superior to breast milk substitutes. Evaluation on survival and bioactivity of lysozyme and lactoferrin in Flash-heated breast milk is ongoing. These data are anticipated by fall 2007. Pilot studies show Flash-heating inactivates cell-free HIV-1 in breast milk as detected by Reverse Transcriptase activity (preliminary data indicates it also inactivates cell-associated HIV-1). It has limited impact on the various vitamins, proteins and antibodies present in breast milk.
In our recent study of 38 milk samples from South Africa, unheated vs. Flash-heated milk had a significantly higher number of samples with bacterial growth at each time point and rate of bacterial growth over 8 hrs. Our data suggest storage of Flash-heated breast milk at room temperature is safe for up to 8 hrs95. We also investigated Flash-heat's effect on breast milk's bacteriostatic activity by spiking heated and unheated milk with 1 x 106 CFU/ml of S. aureus or E. coli as challenge organisms and found no significant impact from Flash-heat on bacteriostatic activity.
Implementing Flash-heat as an infant feeding option
Our data suggest that Flash-heat is a safe method of supplying breast milk to vulnerable HIV-exposed infants. As such, it should be included in comprehensive infant feeding counseling. We have found, however, that most infant feeding counselors are aware of heat treatment, yet despite the fact that it is a WHO recommendation, the very mothers in their clinics say they have never been told of it as an option. Clearly, there is an ethical requirement to provide mothers, such as Rebecca, the information they need so they are equipped to make their own fully informed decision. After all, it was HIV infected mothers in Africa, like Rebecca, that initially inspired this investigation of heat-treating breast milk; they asked what they could do to make their breast milk safer - could they heat it? They wanted a mother-controlled feeding alternative.
Field studies are currently in progress to determine the feasibility of mothers implementing Flash-heat in their homes, although there has been much anecdotal evidence that mothers in various areas of Africa are already heat treating their breast milk. We hope to identify the challenges mothers may face in order to improve implementation strategies.
Flash-heated breast milk is not the panacea to end all postnatal transmission of HIV. But it could reduce the risk for some infants. With the stakes so high, can we afford to ignore any potential option to reduce the burden of children infected with HIV?
Acknowledgements:
Funding for this project has been provided by the National Institute of Child Health and Human Development (Grant #HD051473-01), Thrasher Research
Fund, James B. Pendleton Charitable Trust, North-Central California Center for AIDS Research (an NIH funded program, #P30-AI49366-01), and the University of California at Davis Children's Miracle Network. We gratefully acknowledge Lindiwe Sibeko at the School of Dietetics and Human Nutrition, McGill University for assisting with the writing of this manuscript. We
especially thank the mothers who volunteered to donate breastmilk for this study and the Cato Manor Clinic staff for their time and dedication.
References
1. Shisana O. South African national HIV prevalence, HIV incidence, behavior and communication survey. Pretoria, South Africa: Human Sciences Research Council, 2005.
2. UNAIDS/WHO. AIDS epidemic update. UNAIDS/05.19E. Geneva: UNAIDS, 2005.
3. De Cock KM, Fowler MG, Mercier E, de Vincenzi I, Saba J, Hoff E, Alnwick DJ, Rogers M, Shaffer N. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA 2000; 283(9):1175-82.
4. Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, Sherman J, Bakaki P, Ducar C, Deseyve M, Emel L, Mirochnick M, Fowler MG, Mofenson L, Miotti P, Dransfield K, Bray D, Mmiro F, Jackson JB. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 1999; 354(9181):795-802.
5. Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, Allen M, Nakabiito C, Sherman J, Bakaki P, Owor M, Ducar C, Deseyve M, Mwatha A, Emel L, Duefield C, Mirochnick M, Fowler MG, Mofenson L, Miotti P, Gigliotti M, Bray D, Mmiro F. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet 2003; 362(9387):859-68.
6. Mofenson LM, McIntyre JA. Advances and research directions in the prevention of mother-to-child HIV-1 transmission. Lancet 2000; 355(9222):2237-44.
7. UNAIDS/WHO. AIDS epidemic update: December 2002. Geneva: UNAIDSWHO, 2002.
8. Rousseau CM, Nduati RW, Richardson BA, John-Stewart GC, Mbori-Ngacha DA, Kreiss JK, Overbaugh J. Association of Levels of HIV-1-Infected Breast Milk Cells and Risk of
Mother-to-Child Transmission. J Infect Dis 2004; 190(10):1880-8.
9. Coutsoudis A, Pillay K, Kuhn L, Spooner E, Tsai WY, Coovadia HM. Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: prospective cohort study from Durban, South Africa. AIDS 2001; 15(3):379-87.
10. Cebra JJ. Influences of microbiota on intestinal immune system development. Am J Clin Nutr 1999; 69(5):1046S-1051S.
11. Semba RD, Kumwenda N, Hoover DR, Taha TE, Quinn TC, Mtimavalye L, Biggar RJ, Broadhead R, Miotti PG, Sokoll LJ, van der Hoeven L, Chiphangwi JD. Human immunodeficiency virus load in breast milk, mastitis, and mother- to-child transmission of human immunodeficiency virus type 1.
J Infect Dis 1999; 180(1):93-8.
12. Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, Moulton LH, Ward BJ, Humphrey JH. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. Aids 2005; 19(7):699-708.
13. Leroy V, Becquet R, Rouet F, Ekouevi DK, Viho I, Bequet L, Sakaravitch C, Towne-Gold B, Timite-Konan M, Dabis F. Postnatal transmission risk according to feeding modalities in children born to HIV-infected mothers in a PMTCT project in Abidjan, Côte d'Ivoire. DITRAME PLUS project ANRS 1201/1202. Abstract MoPpB2007. XV International AIDS Conference 2004, Bangkok, Thailand.
14. Coutsoudis A, Dabis F, Fawzi W, Gaillard P, Haverkamp G, Harris DR, Jackson JB, Leroy V, Meda N, Msellati P, Newell ML, Nsuati R, Read JS, Wiktor S. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis 2004; 189(12):2154-66. Epub 2004 May 26.
15. Wiktor SZ, Ekpini E, Karon JM, Nkengasong J, Maurice C, Severin ST, Roels TH, Kouassi MK, Lackritz EM, Coulibaly IM, Greenberg AE. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Cote d'Ivoire: a randomised trial. Lancet 1999; 353(9155):781-5.
16. Kilewo C, Kalrlsson K, Massawe A, Lyamuya E, Lipyoga R, Msemo G, Swai A, Hamud N, Kalokola F, Mhalu F, Biberfeld G. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating infants prophylactically with lamivudine in Dar es Salaam, Tanzania. XVI International AIDS Conference 2006, Toronto, Canada.
17. Vyankandondera J, Luchters S, Hassink E, Pakker N, Mmiro F, Okong P, Kituuka P, Ndugwa C, Mukanka N, Beretta A, Imperiale M, Loeliger E, Giuliano M, Lange J. Reducing risk of HIV-1 transmission from mother to infant through breastfeeding using antiretroviral prophylaxis in infants (Simba study). XVI International AIDS Conference 2006, Toronto, Canada.
18. Shetty AK, Coovadia HM, Mirochnick MM, Maldonado Y, Mofenson LM, Eshleman SH, Fleming T, Emel L, George K, Katzenstein DA, Wells J, Maponga CC, Mwatha A, Jones SA, Abdool Karim SS, Bassett MT. Safety and trough concentrations of nevirapine prophylaxis given daily, twice weekly, or weekly in breast-feeding infants from birth to 6 months. J Acquir Immune Defic Syndr 2003; 34(5):482-90.
19. Kilewo C, Karlsson K, Ngarina M, Massawe A, Lyamuya E, Lipyoga R, Msemo G, Swai A, Mhalu F, Biberfeld G. Prevention of mother to child transmission of HIV-1 through breastfeeding by treating mothers prophylactically with triple antiretroviral therapy in Dar es Salaam, Tanzania * the MITRA Plus study, Abstract #TuAX101. Fourth International AIDS Society Conference on HIV Treatment and Pathogenesis 2007, Sydney, Australia.
20. Arendt V, Ndimubanzi P, Vyankandondera J, Ndayisaba G, Muganda J, Courteille O, Rutanga C, Havuga E, Dhont N, Mujawamassiga A, Omes C, Peltier A. AMATA study: effectiveness of antiretroviral therapy in breastfeeding mothers to prevent post-natal vertical transmission in Rwanda. Abstract #TuAX102. Fourth International AIDS Society Conference on HIV Treatment and Pathogenesis 2007, Sydney, Australia.
21. Kantor R, Katzenstein DA, Efron B, Carvalho AP, Wynhoven B, Cane P, Clarke J, Sirivichayakul S, Soares MA, Snoeck J, Pillay C, Rudich H, Rodrigues R, Holguin A, Ariyoshi K, Bouzas MB, Cahn P, Sugiura W, Soriano V, Brigido LF, Grossman Z, Morris L, Vandamme AM, Tanuri A, Phanuphak P, Weber JN, Pillay D, Harrigan PR, Camacho R, Schapiro JM, Shafer RW. Impact of HIV-1 subtype and antiretroviral therapy on protease and reverse transcriptase genotype: results of a global collaboration. PLoS Med 2005; 2(4):e112.
22. Lee EJ, Kantor R, Zijenah L, Sheldon W, Emel L, Mateta P, Johnston E, Wells J, Shetty AK, Coovadia H, Maldonado Y, Jones SA, Mofenson LM, Contag CH, Bassett M, Katzenstein DA. Breast-milk shedding of drug-resistant HIV-1 subtype C in women exposed to single-dose nevirapine.
J Infect Dis 2005; 192(7):1260-4.
23. Pillay K, Coutsoudis A, York D, Kuhn L, Coovadia HM. Cell-free virus in breast milk of HIV-1-seropositive women. J Acquir Immune Defic Syndr 2000; 24(4):330-6.
24. Chantry CJ, Morrison P, Panchula J, Rivera C, Hillyer G, Zorilla C, Diaz C. Effects of lipolysis or heat treatment on HIV-1 provirus in breast milk. J Acquir Immune Defic Syndr 2000; 24(4):325-9.
25. World Health Organization, UNAIDS. Progress on global access to HIV antiretroviral therapy: a report on "3x5" and beyond In: WHO, ed. Geneva, Switzerland, 2006.
26. WHO, UNICEF, WHO. HIV and Infant Feeding. Guidelines for decision-makers. Geneva: WHO, 1998.
27. Papathakis P, Rollins N. Are WHO/UNAIDS/UNICEF-recommended replacement milks for infants of HIV-infected mothers appropriate in the South African context? Bulletin of the WHO 2004; 82(3):164-171.
28. Coutsoudis A. Breastfeeding and HIV Transmission. Public Health Issues in Infant and Child Nutrition. ed. Robert E. Black and Kim Fleischer Michaelsen. Nestle Nutrition Workshop Series, 2002: 147-166.
29. Coovadia HM, Coutsoudis A. Problems and advances in reducing transmission of hiv-1 through breastfeeding in developing countries. AIDScience 2001; 1(4):1-12.
30. Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, Ndinya-Achola J, Bwayo J, Onyango FE, Hughes J, Kreiss J. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA 2000; 283(9):1167-74.
31. Coutsoudis A, Goga AE, Rollins N, Coovadia HM. Free formula milk for infants of HIV-infected women: blessing or curse? Health Policy Plan 2002; 17(2):154-60.
32. Hardy E, Santos LC, Osis MJ, Carvalho G, Cecatti JG, Faundes A. Contraceptive use and pregnancy before and after introducing lactational amenorrhea (LAM) in a postpartum program. Adv Contracept 1998; 14(1):59-68.
33. Vekemans M. Postpartum contraception: the lactational amenorrhea method. European Journal of Contraception and Reproductive Health Care 1997; 2(2):105-11.
34. WHO Collaborative Study Team. Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet 2000; 355:451-55.
35. UNICEF. State of the World's Children. New York: UNICEF 2000, 2000.
36. WHO. Nutrition risk factors throughout the life course. Nutrition for Health and Development.
37. Victora CG, Smith PG, Vaughan JP, Nobre LC, Lombardi C, Teixeira AM, Fuchs SM, Moreira LB, Gigante LP, Barros FC. Evidence for protection by breast-feeding against infant deaths from infectious diseases in Brazil. Lancet 1987; 2(8554):319-22.
38. Bahl R, Frost C, Kirkwood BR, Edmond K, Martines J, Bhandari N, Arthur P. Infant feeding patterns and risks of death and hospitalization in the first half of infancy: multicentre cohort study. Bull World Health Organ 2005; 83(6):418-426.
39. Nduati R, Richardson BA, John G, Mbori-Ngacha D, Mwatha A, Ndinya-Achola J, Bwayo J, Onyango FE, Kreiss J. Effect of breastfeeding on mortality among HIV-1 infected women: a randomised trial. Lancet 2001; 357(9269):1651-5.
40. Mbori-Ngacha D, Nduati R, John G, Reilly M, Richardson B, Mwatha A, Ndinya-Achola J, Bwayo J, Kreiss J. Morbidity and mortality in breastfed and formula-fed infants of HIV-1- infected women: A randomized clinical trial. JAMA 2001; 286(19):2413-20.
41. Creek T, Luo C, Quick T. HIV-exposed children highly affected by deadly outbreak of diarrhea and severe acute malnutrition - Botswana, 2006. Late Breaker Abstract #1. The 2006 HIV/AIDS Implementers Meeting of the President's Emergency Plan for AIDS Relief 2006, Durban, South Africa.
42. Creek T, Arvelo W, Kim A, Lu L, Bowen A, Mach O, Finkbeiner T, Zaks L, Masunge J, Davis M. A large outbreak of diarrhea among non-breastfed children in Botswana 2006 - Implications for HIV prevention strategies and child health. XIV Conference on Retroviruses and Opportunistic Infections 2007, Los Angeles, CA.
43. Creek T, Arvelo W, Kim A, Lu L, Bowen A, Finkbeiner T, Zaks L, Masunge J, Shaffer N, Davis M. Role of infant feeding and HIV in a severe outbreak of diarrhea and malnutrition among young children, Botswana, 2006. XIV Conference on Retroviruses and Opportunistic Infections 2007, Los Angeles, CA.
44. Morrison P. HIV and infant feeding: to breastfeed or not to breastfeed: the dilemma of competing risks. Part 1. Breastfeed Rev 1999; 7(2):5-13.
45. Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. Influence of infant-feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa: a prospective cohort study. South African Vitamin A Study Group.
Lancet 1999; 354(9177):471-6.
46. Hanson LA, Ahlstedt S, Carlsson B, Fallstrom SP, Kaijser B, Lindblad BS, Akerlund AS, Eden CS. New knowledge in human milk immunoglobulin. Acta Paediatr Scand 1978; 67(5):577-82.
47. Lonnerdal B. Biochemistry and physiological function of human milk proteins. Am J Clin Nutr 1985; 42(6):1299-317.
48. Udall JN, Colony P, Fritze L, Pang K, Trier JS, Walker WA. Development of gastrointestinal mucosal barrier. II. The effect of natural versus artificial feeding on intestinal permeability to macromolecules. Pediatr Res 1981; 15(3):245-9.
49. Piwoz E, Iliff P, Tavengwa N, Zunguza C, Marinda E, Nathoo K, Moulton L, Ward B, Humphrey J. Early introduction of non-human milk and solid foods increases the risk of postnatal HIV-1 transmission in Zimbabwe. XV International AIDS Conference 2004, Bangkok, Thailand.
50. Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr 1999; 69(5):1035S-1045S.
51. Kuhn L, Stein Z. Infant survival, HIV infection, and feeding alternatives in less- developed countries. Am J Public Health 1997; 87(6):926-31.
52. Smith MM, Kuhn L. Infant-feeding patterns and HIV-1 transmission. Lancet 1999; 354(9193):1903-4.
53. Ross JS, Labbok MH. Modeling the effects of different infant feeding strategies on infant survival and mother-to-child transmission of HIV. Am J Public Health 2004; 94(7):1174-80.
54. Fowler MG, Newell ML. Breast-feeding and HIV-1 transmission in resource-limited settings. J Acquir Immune Defic Syndr 2002; 30(2):230-9.
55. WHO. Consensus Statement: Held on behalf of the Inter-Agency Task Team (IATT) on Prevention of HIV Infections in Pregnant Women, Mothers and their Infants. WHO HIV and Infant Feeding Technical Consultation 2006, Geneva.
56. WHO, UNICEF. HIV and infant feeding: A guide for health-care managers and supervisors. Geneva, Switzerland: WHO, 2004.
57. Wilfert CM, Ammann A, Bayer R, Curran JW, del Rio C, Faden RR, Feinberg MB, Karlin PD, Levine RJ, Luo C, Sessions K. Science, ethics, and the future of research into maternal infant transmission of HIV-1: Consensus statement. Lancet North Am Ed 1999; 353(9155):832-5.
58. Coovadia H, Coutsoudis A, Rollins N, Bland JM, Newell ML. Prevention of HIV transmission from breastfeeding. XIV Conference on Retroviruses and Opportunistic Infections 2007, Los Angeles, CA.
59. Piwoz E, Kasonde P, Vwalika C, Shutes E, Sinkala M, Kankasa C, Aldrovandi G, Kuhn L, Thea D. The feasibility of early rapid breastfeeding cessation to reduce postnatal transmission of HIV in Lusaka, Zambia. XVI International AIDS Conference 2006, Toronto, Canada.
60. De Paoli MM, Mkwanazi DB, Rollins NC. Rapid cessation of breastfeeding - a safe and feasible PMTC strategy? XVI International AIDS Conference 2006, Toronto, Canada.
61. Johnson W, Alons C, Fidalgo L, Piwoz E, Kahn S, Macombe A, Catarina R, Briend A, Lovich R, Warming E, Floriano F, Chavane V. The challenge of providing adequate infant nutrition following early breastfeeding cessation by HIV-positive, food-insecure Mozambican mothers. XVI International AIDS Conference 2006, Toronto, Canada.
62. Becquet R, Leroy V, Ekouevi DK, Viho I, Castetbon K, Fassinou P, Dabis F, Timite-Konan M. Complementary feeding adequacy in relation to nutritional status among early weaned breastfed children who are born to HIV-infected mothers: ANRS 1201/1202 Ditrame Plus, Abidjan, Cote d'Ivoire. Pediatrics 2006; 117(4):e701-10.
63. Kourtis AP, Fitzgerald G, Hyde L, Tien H, Chavula C, Mumba N, Magawa M, Knight R, Chasela C, van der Horst C, team Bs. Diarrea in uninfected infants of HIV-infected mothers who stop breastfeeding at 6 months: the BAN study experience. XIV Conference on Retroviruses and Opportunistic Infections 2007, Los Angeles.
64. Kafulafula G, Thigpen M, Hoover D, Li Q, Kumwenda N, Mipando L, Taha T, Mofenson L, Fowler MG. Post-weaning gastroenteritis and mortality in HIV-uninfected African infants receiving antiretroviral prophylaxis to prevent MTCT of HIV-1. XIV Conference on Retroviruses and Opportunistic Infections 2007, Los Angeles, CA.
65. Sinkala M, Kuhn L, Kankasa C, Kasonde P, Vwalika C, Mwiya M, Scott N, Semrau K, Aldrovandi G, Thea D, Group ZS. No benefit of early cessation of breastfeeding at 4 months on HIV-free survival of infants born to HIV-infected mothers in Zambia: The Zambia exclusive breastfeeding study. XIV Conference on Retroviruses and Opportunistic Infections 2007, Los Angeles, CA.
66. Thomas T, Masaba R, van Eijk A, Nasokho P, Thigpen M, Fowler MG. Rates of diarrhea associated with early weaning among infants in Kisumu, Kenya. XIV Conference on Retroviruses and Opportunistic Infections 2007, Los Angeles, CA.
67. Onyango C, Mmiro F, Bagenda D, Mubiru M, Musoke P, Fowler MG, Jackson J, Guay LA. Early breastfeeding cessation among HIV-exposed negative infants and risk of serious gastroenteritis: findings from a peri-natal prevention trial in Kampala, Uganda. XIV Conference on Retroviruses and Opportunistic Infections 2007, Los Angeles, CA.
68. Thea DM, Aldrovandi G, Kankasa C, Kasonde P, Decker WD, Semrau K, Sinkala M, Kuhn L. Post-weaning breast milk HIV-1 viral load, blood prolactin levels and breast milk volume. Aids 2006; 20(11):1539-1547.
69. Coutsoudis A. Infant Feeding Dilemmas Created by HIV: South African Experiences. J Nutr 2005; 135(4):956-9.
70. Coutsoudis A. Promotion of exclusive breastfeeding in the face of the HIV pandemic. Lancet 2000; 356(9242):1620-1.
71. Hankins C. Preventing mother-to-child transmission of HIV in developing countries: recent developments and ethical implications. Reprod Health Matters 2000; 8(15):87-92.
72. Latham MC, Preble EA. Appropriate feeding methods for infants of HIV infected mothers in sub- Saharan Africa. BMJ 2000; 320(7250):1656-60.
73. Rollins N, Meda N, Becquet R, Coutsoudis A, Humphrey J, Jeffrey B, Kanshana S, Kuhn L, Leroy V, Mbori-Ngacha D, McIntyre J, Newell ML. Preventing Postnatal Transmission of HIV-1 Through Breast-feeding: Modifying Infant Feeding Practices. J Acquir Immune Defic Syndr 2004; 35(2):188-195.
74. Tomlinson RJ, Madjarov A. Global voices on HIV/AIDS. Pasteurised human breast milk should be considered. BMJ 2002; 324(7344):1034-5.
75. WHO, UNICEF, USAID, URC/QAP, Health TMo, TFNC, KCMC, COUNSENUTH, AXIOS. HIV and Infant Feeding: A question and answer guide for counselors. 2005.
76. Orloff SL, Wallingford JC, McDougal JS. Inactivation of human immunodeficiency virus type I in human milk: effects of intrinsic factors in human milk and of pasteurization. J Hum Lact 1993; 9(1):13-7.
77. Lawrence R, Lawrence R. Breastfeeding: A guide for the medical profession. 6th ed. Philadelphia, Pennsylvania: Elsevier Mosby, 2005.
78. Eglin RP, Wilkinson AR. HIV infection and pasteurisation of breast milk. Lancet 1987;1(8541):1093.
79. McDougal JS ML, Cort SP, et al. Thermal inactivation of the acquired immunodeficiency syndrome virus, human T lymphotropic virus-III/lymphadenopathy-associated virus, with special reference to antihemophilic factor. J Clin Invest 1985; 76(2):875-7.
80. Behringer R, Kessler HG. Influence of individual milk consituents on the heat resistance of Bacillus licheniformis and Bacillus stearothermophilus spores. Int Dairy Journal 1992; 2:243-255.
81. Jagannath A, Tsuchido T. Validation of a polynomial regression model: the thermal inactivation of Bacillus subtilis spores in milk. Lett Appl Microbiol 2003; 37(5):399-404.
82. Israel-Ballard K, Chantry CJ, Padian N. Zimbabwean attitudes and resource accessibility as a measure of the feasibility and acceptability of heat treating expressed breast milk for prevention of mother to child transmission of HIV. American Public Health Association 130th Annual Meeting 2002, Philadelphia, PA.
83. WHO/UNICEF/UNAIDS. HIV and infant feeding counseling: a training course - participants' manual. 2000.
84. Jeffery BS, Webber L, Mokhondo KR, Erasmus D. Determination of the effectiveness of inactivation of human immunodeficiency virus by Pretoria pasteurization. J Trop Pediatr 2001; 47(6):345-9.
85. Jeffery BS, Mercer KG. Pretoria pasteurisation: a potential method for the reduction of postnatal mother to child transmission of the human immunodeficiency virus. J Trop Pediatr 2000; 46(4):219-23.
86. Israel-Ballard K, Maternowska C, Abrams B, Morrisson P, Chitibura L, Chipato T, Chirenje Z, Padian N, Chantry C. Acceptability of heat-treating breast milk to prevent mother-to-child transmission of HIV in Zimbabwe: A qualitative study. J Hum Lact 2006; 22(1):48-60.
87. Goldblum RM, Dill CW, Albrecht TB, Alford ES, Garza C, Goldman AS. Rapid high-temperature treatment of human milk. J Pediatr 1984; 104(3):380-5.
88. Morgan JN, Lin FJ, Eitenmiller RR, Barnhart HM, Toledo RT. Thermal destruction of Eschericihia coli and Klebsiella pneumoniae in human milk. J Food Pro 1988; 51:132-136.
89. Dhar J, Fichtali J, Skura BJ. Pasteurization Efficiency of a HTST System for Human Milk. J Food Sci 1996; 61(3):569-573.
90. Terpstra F, Rechtman DJ, Lee ML, Van Hoeij K, Berg H, Van Engelenberg F, Van't Wout AB. Antimicrobial and antiviral effectof high-temperature short-time (HTST) pasteurization applied to human milk. Breastfeeding Medicine 2007; 2(1):27-33.
91. Israel-Ballard K, Chantry C, Dewey K, Lonnerdal B, Sheppard H, Donovan R, Carlson J, Sage A, Abrams B. Viral, Nutritional, and Bacterial Safety of Flash-Heated and Pretoria-Pasteurized Breast Milk to Prevent Mother-to-Child Transmission of HIV in Resource-Poor Countries: A Pilot Study. J Acquir Immune Defic Syndr 2005; 40(2):175-181.
92. Israel-Ballard K, Donovan R, Chantry C, Coutsoudis A, Sheppard H, Sibeko L, Abrams B. Flash-heat inactivation of HIV-1 in human milk: a potential method to reduce postnatal transmission in developing countries. J Acquir Immune Defic Syndr 2007; 45(3):318-23.
93. Israel-Ballard K, Abrams B, Coutsoudis A, Sibeko L, Chantry C. Vitamin Content of Flash-heated Breast Milk as an Infant Feeding Option for HIV Positive Mothers in Developing Countries. . Pediatric Academic Society Annual Meeting 2007, Toronto, Canada.
94. Chantry C, Israel-Ballard K, Moldoveanu Z, Peerson JM, Coutsoudis A, Sibeko L, Abrams B. Effect of Flash-heat on Immunoglobulins in Breast Milk. . Pediatric Academic Society Annual Meeting 2007, Toronto, Canada.
95. Israel-Ballard K, Coutsoudis A, Chantry CJ, Sturm AW, Karim F, Sibeko L, Abrams B. Bacterial safety of flash-heated and unheated expressed breast milk during storage. J Trop Pediatr 2006; 52(6):399-405.