Author: Michael Rosenthal
Institution: Department of Biology, Tufts University
Date: September 2006
ABSTRACT
The tropical forest canopy is a unique ecosystem with complex environmental interactions, which allows for a high level specialization of insects. The purpose of this study was to evaluate whether in creased species specialization has created species variation between nocturnal and diurnal canopy insects. Insects were collected from six trees using suspended traps containing three types of bait (carrion, rotten fruit, and scents). Within the diurnal and nocturnal traps, insect diversity and species richness was quantified for the three baits, and for number of insects collected. The total number of Lepidoptera, Coleoptera, Diptera, Hymenoptera, and Orthroptera collected was 581; 397 were found diurnally and 184 nocturnally. The total number of insects found for each bait was 48 for scent baits (37 diurnal, 11 nocturnal); 115 for fruit baits (83 diurnal, 32 nocturnal) and 416 for meat baits (276 diurnal, 140 nocturnal). Diurnal traps had significantly higher diversity than nocturnal traps higher in the diurnal traps for meats and scents (ie. diurnal meat traps versus nocturnal meat traps and diurnal scent traps versus nocturnal scent traps). In contrast, nocturnal insect diversity was significantly greater for fruit traps. The diurnal traps had a significantly higher overall abundance, species richness, and diversity of insects than nocturnal traps in the tropical forest canopy.
INTRODUCTION
It is readily accepted that animal communities contain specialized, similar species, with narrow environmental niches. In 1967, MacArthur and Wilson explored the niche compression hypothesis, which states that as species become "packed" into a community, the habitat occupied by each species shrinks. Indeed, Basset (1996) found 94 different species of herbivorous leaf chewing insects in a single tree in New Guinea, demonstrating high insect specialization, species richness and diversity. Similar studies done by Marquis and Braker (1994) at La Selva, demonstrated a high specificity of butterflies and acridid grasshoppers in a wet tropical site, which allowed for diversity and species richness within a compact community.
It is believed that diversity evolves through niche specialization and resource partitioning (Rosmoser and Stoffolano, 1998). What is not known is if these organisms specialize in a temporal manner, where some organisms can exploit resources at night (nocturnal feeders) and others during the day (diurnal feeders). As there is high diversity and species richness in the tropical rainforest, a forest canopy should be no different. The diversity and abundance of insects in tropical tree crowns is enormous. A study by J. Longino (1994) found a diverse ant population within the canopy on Barro Colorado Island, Panama. Although not much is known about insect populations within tropical forest canopies, studies indicate there is a high level of diversity.
The purpose of this study was to determine if there is a difference in species richness, diversity, and abundance of nocturnal versus diurnal canopy insects on contrasting resources (baits).
MATERIALS AND METHODS
Field Site
This study was carried out in the forest sub-canopy of La Estación Biológica de Monteverde, Puntarenas, Costa Rica between July 19 July 29, 2004. Following one of the Quebrada Máquina River tributaries near the station, six different trees of two species, Conostegia oeristediana (Melastomatceae) and Cinnamomum sp. (Lauraceae), were used for sampling. All trees were located at similar elevations (1450-1460m) within the lower montane wet forest life zone (Haber, 1991), and exhibited similar height and diameter at breast height. Trees were at least 60 meters apart to insure sample independence. Diurnal and nocturnal precipitation and temperature measurements were provided. The rainfall accumulation at night was greater (71.3 cm) than during the day (53.3 cm). On average, there was a 5.5 degree Celsius temperature change from day to night during the observation period (19 to the 29 of July, 2004). The mean daytime temperature was 21.2 degrees Celsius, while the mean nocturnal temperature was 15.7 degrees Celsius.
Insect Trap Design and Baits
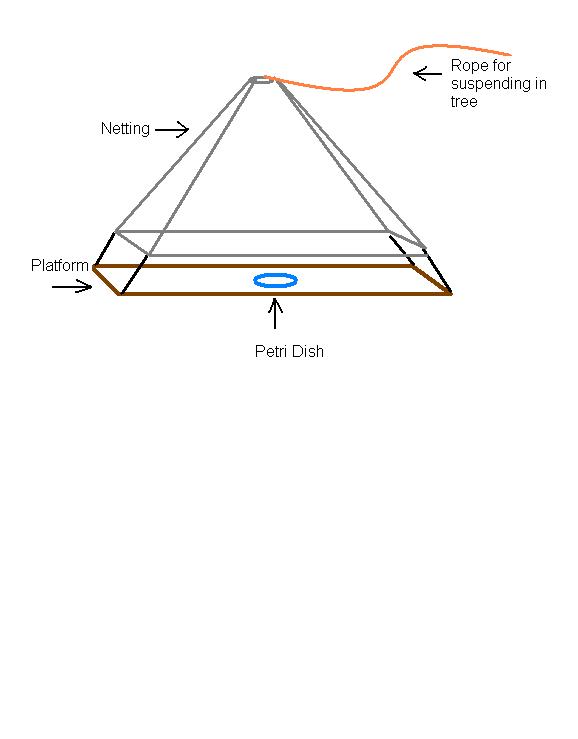
Three types of baits were used: fruit, carrion, and artificial scents. The fruit bait consisted of banana segments placed in the center of the traps on Petri dishes, which were fastened to the platform. Eucalyptus scent and a floral scent were placed on filter paper and thumb tacked to the center of the platform. Fresh beef cubes were placed on a Petri dish at the center. The same baits were used throughout the experiment and replaced as necessary to maintain freshness. Each tree contained three traps, each containing different bait.
The traps consisted of a board platform attached to a hanging screen of mosquito netting, which was suspended in a tree. Two wire rings, a foot in diameter, were used at the top and bottom of the trap to afford stability. The mosquito netting was draped over one ring at the top and secured to the bottom ring. The platforms were 30 cm by 30 cm and protruded from all sides of the trap with the net positioned in the middle. Petri dishes were secured to the middle of the platform as a retainer for the meat and fruit. Thumbtacks were used to fasten the scented filter paper to the platform.
Sampling Technique
The traps were checked at twelve-hour intervals (dusk and dawn). Ten nocturnal and ten diurnal samples were collected. Although no specific tree was utilized as a control, each of the trees studied were situated approximately 100 yards apart in order to establish independence. The total number of specimens was recorded for each site, separated by time of collection (nocturnal or diurnal), and by bait type. Collection occurred by lowering the traps, collecting the insects by hand, and placing them in bags according to which tree and trap each insect was found in. Specimens were brought to the station for identification. Species were separated based on morphological features and later identified to family using field guides and the station's reference collections of pinned insects (Solis 1990, Zumbado 1999, Ugalde 2002, Arnett and Jacques 1981).
Data Analysis
The Shannon-Weiner index to assess diversity and the Jaccard similarity index were used to determine the overlap between nocturnal and diurnal insects. A student's modified t-test was used to compare the diversity indices between the diurnal and nocturnal insects overall and within each trap (Abramson, (1994).
RESULTS
Twenty-seven different morpho-species were identified that belonged to five different Orders and twenty-two different Families (Appendix B). Dipterans exhibited the highest number of species (8), while Coleopterans exhibited the greatest abundance overall (288 individuals). Twenty-four diurnal morpho-species and 21 nocturnal morpho-species were recorded (Fig.1). Diurnal and nocturnal species richness differed among baits: the meat traps had 15 diurnal species vs. 9 nocturnal species, the fruit traps had 15 species vs. 14 species, and the scents had 9 vs. 2 species (Fig. 2).
article_778_order_3
article_778_order_4
The total number of insects collected was 581: 397 were found diurnally and 184 nocturnally. The total number of insects found for each bait was 48 for scent bait (37 diurnal, 11 nocturnal); 115 for fruit bait (83 diurnal, 32 nocturnal) and 416 for fruit bait (276 diurnal, 140 nocturnal) (Fig. 3).
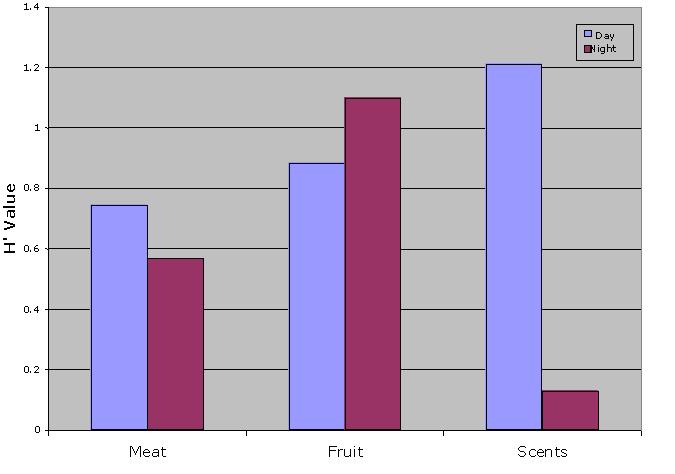
Diurnal traps had significantly higher diversity (H' = 1.000) than nocturnal one (H' = 0.863) (Modified t-test, t = 2.651, P < 0.005). Diversity was statistically significantly higher in the diurnal traps for meats and scents: diurnal meat trap (H' = 0.746) and nocturnal meat trap (H' = 0.570) (Modified t-test, t (n 1) = 3.266, P < 0.001), diurnal scents trap (H' = 0.763) and nocturnal scents trap (H' = 0.132) (Modified t-test, t = 5.968, P < 0.001) (Fig. 4). Nocturnal insect diversity was significantly greater for fruit traps: diurnal fruit trap (H' = 0.885) and nocturnal fruit trap (H' = 1.100) (Modified t-test, t = 3.016, P < 0.002).
article_778_order_6
There was a moderate level of overlap between species found in diurnal traps and nocturnal traps (Jaccard = 0.59). The overlap between nocturnal and diurnal insects attracted to meat (0.50) and fruit (0.61) was similar, while there was little overlap for scents (0.22).
article_778_order_5
It is evident that there are differences in diversity and species richness between insects found at night and those found during the day (Fig. 5). Diurnal insects exhibited higher species richness and diversity, however, evidence of specialization by the Jaccard index demonstrated a fair amount of overlap between overall night and day sampling (0.593) and between day and night for two of the baits (fruit = 0.61, meat = 0.50)(Fig 4).
DISCUSSION
article_778_order_0
The motivation for this study was to determine if there is a difference in species richness, diversity, and abundance of nocturnal versus diurnal canopy insects in Costa Rican montane rain forests. There are several possibilities which explain why more diurnal insect species were identified and individuals were caught: heavier rain falls at night, temperature fluctuation between night and day, foraging strategies of diurnal and nocturnal insects, effectiveness of traps catching and retaining insects, and bias of baits. Sampling was performed during the rainy season in Costa Rica, which has implications for flying insects found in Lepidoptera, Coleoptera, Diptera, Hymenoptera, and Orthroptera as rain often hinders flight (Huffaker and Gutierrez, 1999). Since the majority of insects trapped were flying insects, the heavier nocturnal rainfall could explain the diurnal variation in species richness, diversity, and abundance.
In tropical zones, the most significant temperature fluctuations are not seasonal, but rather diurnal and nocturnal. The differences in temperature between day and night could also explain the diversity and species richness between diurnal and nocturnal insects, as the insects must expend more energy in order to adapt to the lower nocturnal temperatures. Indeed, at the individual level the available heat, as indicated by body temperature, is the most significant variable that determines growth and activity (Huffaker and Gutierrez, 1999). Metabolic activities essential for development, feeding, dispersal, reproduction, and survival may all are impeded by the decrease in nocturnal temperature, which likely results in greater diurnal diversity, species richness, and abundance.
Diurnal insects become more active when the sun heats their bodies, while nocturnal insects rely on stored body energy. Morris (1967) demonstrated that during colder seasonal temperatures, the webworm, Hypahntria cunea's development is retarded and the larvae must feed on older foliage, which forces a late emergence from the larval stage and a decrease in numbers.
Nocturnal foraging may also influence diversity and species richness. The "cost" of nocturnal foraging is greater in terms of calories used for flying, so the "rewards" must be higher (Prince, 1997). Heinrich (1979) illustrated the importance of maintaining sufficiently high body temperature to allow efficient flight. For example, a bumblebee cannot fly if its muscle temperature drops below 30 degrees Celsius. Foraging at night during cooler temperatures is usually performed by larger insects capable of temperature regulation (Prince, 1997), which has implications for nocturnal insects that do not benefit from solar radiation. As the nocturnal insects were generally larger, they may have been too big for the trap opening, excluding them from the samples. The traps were designed to attract insects to the platform using bait, leading them to fly upwards when leaving and thus trapping themselves. This worked well for the larger insects, such as the Lepidoptera and most Diptera, but some smaller insects possibly escaped collection as well.
The baits used in this experiment may have created a bias toward diurnal insects as rotting fruit, carrion, and scents produce a smell. Diurnal insects are more attracted by visual and olfactory stimuli than nocturnal insects. Nocturnal insects on the other hand, are more attracted by chemical and olfactory cues than visual and, therefore, may have been more able to locate the baits. Interestingly, the fruit traps, had a significantly greater nocturnal (H' = 1.100) than diurnal (H' = 0.885) diversity, which may suggest that the fruit bait was biased towards nocturnal insects. The diversity of the insects at night (32), was more evenly distributed amongst the 15 different morpho-species when compared to that of diurnal fruit insects (83), where the majority were within the 14 morpho-species found in two Families: an ant belonging to the Formicidae (11 individuals) and a fly belonging to Tephritidae (34 individuals).
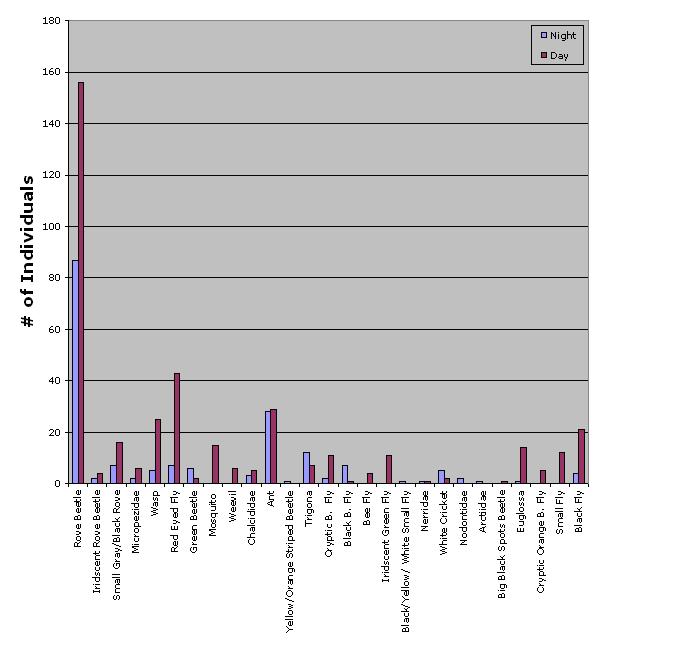
The abundance of Rove Beetles (Staphylinidae), both diurnal and nocturnal, identified in the meat and fruit traps was interesting. The number of Rove Beetles far exceeded that of any other insect (272 total: diurnal = 182, nocturnal = 97, fruit = 18, and meat = 261) (Fig. 6). Through personal observation, these insects would swarm the meat, covering the bait, devouring it, and their aggressive behavior allowed them to amass in large numbers. Interestingly, Hanski (1990) studied carrion arthropod assemblages in southern Finland and found that increased larval density of one dominant carrion species reduced survival, size, fecundity, and longevity of emerging adults of other species. This indicates that competition has an important influence on population dynamics, similar to that apparent within Rove beetle dynamics.
Rove beetles occur in a variety of habitats: on carrion, on the ground or under objects, near streams and lakes, under bark, in fungi, on flowers, or in decaying plant matter (Borror and White, 1970). With this much adaptation, it is not surprising to find them in the canopy. Although the Rove Beetles were primarily found during the day, it was interesting to find them at night as well. Two species of Staphylinidae have been known to exhibit parental care: Platystethus arenarius and Bledius spectabilis(Hinton, 1944). The female lays its eggs in a chamber and will vigorously protect them from other arthropods, including other Staphylinid adults. This maybe the case for some species in the traps and explains the decrease in abundance of males at night. They may have been present during the day, while the females protecting their eggs may have been present only at night.
The Chalcididae wasp was found only in fruit and scent traps. This insect is not attracted to bait as adult Chalcids are parasitoids, and lay their eggs only on other insects (Borror et al., 1989). The wasp most likely was not attracted to the baits, but rather followed insects into the traps in order to parasitize them.
The results of this study have shown a difference between diurnal and nocturnal species richness and diversity in the tropical montane forest canopy. Further research, however, needs to be done in order to accurately determine what specific factors contribute to these differences. Limitations of this study included a lack of testing at different times during the year to see if climate or weather patterns affect diversity and species richness, and a controlled temperature laboratory experiment to identify if temperature affects the variation observed between diurnal and nocturnal insects in the field. Further experiments using different trees and baits would help clarify which species of insects are affected by diurnal and nocturnal habits. The rich biodiversity of the Costa Rican tropical montane forest canopy offers enormous study opportunities in species richness, diversity, and specialization. This study demonstrated that increased specialization has created species diversity between the nocturnal and diurnal canopy insects.
Acknowledgments
At this time it is important to thank all the wonderful people who have helped make this research possible. Thanks to Carlos Guindon for initiating my interest in Environmental Research. Thanks to Colin Orians for his tireless dedication, understanding, and advice. Lastly, thanks to my family for their support and love.
References
Abramson, J.H. (1994). Making Sense of Data. 2nd Edition. Oxford University Press. New York, New York, USA
Arnett, R. H. Jr. and R.L. Jacques. 1981. Simon and Schuster's guide to insects. Simon and Schuster Inc.,
New York, New York, USA.
Basset, Y. 1996. Local communities of arboreal herbivores in Papua New Guinea: Predictors of insect
variables. JSTOR Ecology 77: 1906-1919.
Borror, D. J., C. A. Triplehorn and N. F. Johnson. 1989. An introduction of the study of insects. Harcourt
Brace College Publishers, Philadelphia, Pennsylvania, USA.
Borror, D. J. and R. E. White. 1970. A field guide to insects of America north of Mexico. Houghton Mifflin
Company, Boston, Massachusetts, USA.
Haber, W. A. 1991. Lista provisional de las plantas de Monteverde, Costa Rica. Brenesia 34: 63-120. In N.
M. Nadkarni and N. T. Wheelright. 2000. Monteverde: Ecology and conservation of a tropical rainforest. Oxford University Press, New York, New York, USA.
Hanski, I. 1990. Living in a patchy environment. Oxford Science Publications, Oxford, UK.
Heinrich, B. 1979. Bumble-bee economics. Harvard Univ. Press, Cambridge, Mass.
Hinton, H. E. 1944. Some general remarks on sub-social beetles, with notes on the biology of the
staphylinid, Platystethus arenarius. Proceedings of the Royal Entomological Society of London, A19: 115-128.
Huffaker, C. B. and A.P. Gutierrez. 1999. Ecological entomology. John Wiley and Sons, Inc., New York, New York, USA.
Longino, J. 2004. Arboreal ant species richness in primary forest, secondary forest, and pasture habitats of a tropical montane landscape. Biotropica, 36(3): 402-409
MacArthur, R. H. and E. O. Wilson. 1967. The theory of island biography. Princeton Univ. Press, Princeton, New Jersey, USA.
Marquis. R.J. and H.E. Braker.1994. 261-281. In L. A. Dade, K. S. Bawa, H. A. Hespenheide, and G. S. Hartshorn. La Selva: Ecology and natural history of a neotropical rain forest. Univ. Chicago Press, Chicago, Illinois, USA.
Morris, R. F. 1967. Influence of parental food quality on the survival of Hyphantria cunea. Can. Entomol. 99: 24-33.
Prince, P.W. 1997. Insect Ecology. John Wiley and Sons, Inc., New York, New York, USA.
Rosmoser, W. S. and J. G Stoffolano. 1998. The science of entomology. WCB/McGraw Hill, Boston, Massachusetts, USA.
Solís, A. 1999. Escarabajos de Costa Rica Beetles. National Institution of Biodiversity, Santo Domingo, Heredia, Costa Rica.
Ugalde, J. A. 2002. Avispas, abejas y hormigas Costa Rica Wasps, bees and ants. National Institution of Biodiversity, Santo Domingo, Heredia, Costa Rica.
Zumbado, M. 1999. Dípteros de Costa Rica Diptera. National Institution of Biodiversity, Santo Domingo, Heredia, Costa Rica.
APPENDIX A
article_778_order_1
APPENDIX B
article_778_order_2