Author: Tang Cristina
Institution: Biochemistry
Date: September 2005
As the average human lifespan has increased, diseases that have higher incidence with increasing age have become a research priority in many labs around the world. One of these age-related diseases is Alzheimer's.
Patients with Alzheimer's disease suffer gradual loss of memory and judgement, and are eventually incapable of performing basic tasks such as eating. One of the physical hallmarks of Alzheimer's is the formation of clusters of proteins between and inside nerve cells of the brain. These protein clumps, called plaques, damage the brain's nerve cells, resulting in the set of symptoms (i.e. memory loss, emotional instability) observed in Alzheimer's patients.
Nowadays, there are drugs that can reduce the severity of the symptoms of Alzheimer's disease in its early stages; but no treatment can prevent or stop the progression of this degenerative disease. However, researchers continue to take on the challenge of unraveling the mysteries locked in the brains of Alzheimer's patients.
article_505_order_0
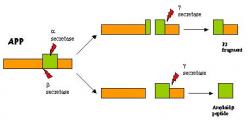
Fifteen years ago, it was found that the main component of the plaques that formed between the nerve cells was a product of the biological processing of a protein called the amyloid precursor protein or APP (1, 2). APP, like many other proteins in the body, goes through a modification step called post-translational processing after it is synthesized by the ribosomes. During post-translational processing, APP is cleaved into smaller fragments, or peptides, by proteins called enzymes. When the two enzymes named a and g secretase make excisions on APP, a harmless fragment, p3, is produced (3). However, if APP is cut by the b and g secretases, then fragments of either 40 or 42 amino acids (the building blocks of proteins) result and are released from the cell (4). The 42 amino acid long fragment, named the amyloid beta peptide , is prone to associate with other amyloid beta peptides and has been deemed responsible for the formation of plaques in Alzheimer's patients.
Under normal conditions, less than 10% of the fragments produced from the cleavage of APP by the b and g secretases are the dangerous amyloid beta peptide. However, mutations in APP (the replacement of a specific amino acid by another one) can increase the production of the 42-amino-acid-long peptide, thus raising the chances of plaque formation around the nerve cells (5).
Because of its involvement in Alzheimer's disease, researchers have studied the structure and the processing of APP intensely. Its biological functions, however, remain poorly understood. For example, scientist don't know why our bodies go to the trouble of making this protein while taking the risk of producing a harmful peptide, and experiments to determine the roles of APP in the cells have not been very successful.
Meanwhile, some researchers have started to turn their attention to another product of the post-translational processing of APP: the cytoplasmic tail of APP, a small peptide that is buried inside the cell after APP is cut by the g secretase. Although this peptide is not yet found to be involved in Alzheimer's disease, it might hold the reasons for the production and cleavage of APP. An understanding of the cellular functions, if any, of this fragment may provide useful information for the prevention and treatment of Alzheimer's disease.
Recently, researchers T. Südhof of the Howard Hughes Medical Institute and X. Cao have found that the cytoplasmic tail of APP together with two other proteins, Fe65 and Tip60, can activate transcription, the process by which genes are "turned on" (6). They proposed two mechanisms by which the cleavage of APP is linked to transcriptional activation. In their first model, APP grabs onto Fe65 tightly, preventing it from entering the nucleus and from binding to Tip60 to activate transcription. When APP is cut into small fragments, the Fe65 is released from APP and is free to enter the nucleus. In the second model, cleavage of APP is necessary because the resulting cytoplasmic fragment is itself required for the activation of transcription (6). Data thus far seem to support the second model; however, further research is needed to confirm this hypothesis.
Cao and Südhof are among the first to uncover a physiological function of APP. As more proteins that interact with the cytoplasmic tail of APP are identified, the list of possible of functions of the peptide gets longer and the cellular network that the peptide seems to be involved in becomes more complex. Many more years of research will be necessary to solve the whole puzzle surrounding APP and the fragments derived from it. Understanding APP through studies of its cytoplasmic tail will give scientists some insight into the causes of Alzheimer's disease, and may even provide a novel way to prevent or treat this degenerative disease.
Suggested Reading
(1) Glenner, G.G., Wong, C. W. (1984) Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120: 885-890
(2) Masters, C.L. et. al. (1985) Amyloid plaque core protein in Alzheimer's disease and Down syndrome. Proc. Nat. Acad. Sci. USA. 82: 4242-4249
(3) Lalowski, M. et. al. (1996) The "nonamyloidogenic" p3 fragment (amyloid beta 17/42) is a major constituent of Down's syndrome cerebellar preamyloid. J.Biol. Chem. 271(52): 33623-31
(4) Lansbury, P.T. (1997) Structural neurology: are seeds at the root of neuronal degeneration? Neuron. 14: 1151-54
(5) Haas, C., et. al. (1994) Mutations associated with a locus for familial Alzheimer's disease result in alternative processing of amyloid beta-protein precursor. J. Biol. Chem. 269: 17741-48
(6) Cao, X., Südhof, T. (2001) A transcriptively active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 293(5527): 115-20