Author: Pattanayak Vikram
Institution: Biochemistry and Biophysics
Date: September 2005
Smallpox, a disease caused by the variola virus, has been absent from the globe for more than 25 years and absent from the United States since 1949. Recent events, however, have led the U.S. government to fear that smallpox could be used in a bioterrorist attack. Due to this possibility, President George Bush announced a plan in December 2002 to vaccinate nearly one million military personnel and healthcare workers against smallpox.
While the immediate threat of smallpox exposure is minimal, a Centers for Disease Control (CDC) website on the vaccination program explains, "The attacks of September and October 2001 have heightened concern that terrorists may have access to the virus and attempt to use it against the American public." The concern about a possible smallpox attack led the government to consider a vaccination program. However, the possible side effects of the vaccine merited careful consideration in developing an appropriate strategy to implement the program with minimal risk.
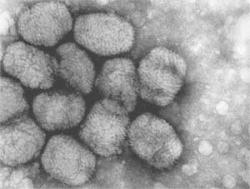
Electron micrograph of variola, the virus that causes smallpox. Source: CDC/Fred Murphy; Sylvia Whitfield.
The government's vaccination initiative centers around Dryvax, the same vaccine used in the United States until 1972, when routine smallpox vaccination ended. Unlike most other vaccines that contain dead virus particles, Dryvax contains a live virus called vaccinia, which is related to smallpox. To administer the vaccine, doctors use a special needle to deliver the vaccinia virus into the top layers of the skin, where it begins to replicate. The body recognizes this infection and generates an immune response, creating antibodies that kill the vaccinia. These antibodies also recognize smallpox, vaccinia's close relative, conferring immunity to the deadly disease.
Vaccinating people against smallpox can be risky. The CDC lists arm soreness, fever, and mild body aches as typical mild side effects of the vaccinia vaccination. One thousand out of every million people vaccinated experience more serious side effects, which include the spread of the vaccinia virus from the vaccination site to other parts of the body or to other people if they touch the vaccination site before it has healed.
Though not as harmful as smallpox, vaccinia does carry the risk of potentially life threatening side effects. According to the CDC, out of every million people exposed, between 14 and 52 will have life-threatening side effects, and one to two of them will die. In some cases, these serious side effects can be eased with Vaccinia Immune Globulin (VIG), which contains antibodies to the vaccinia virus isolated from people who have been vaccinated multiple times. These pre-made antibodies help the body mount an immune response and fight off vaccinia.
Smallpox vaccination kit. Dryvax smallpox vaccine with diluent (top) and bifurcated needle for delivery (bottom). Source: CDC.
While the smallpox vaccine can result in the spread of the vaccinia virus to others, health officials stress that vaccination cannot lead to the transmission of smallpox to the general public. "The smallpox vaccine does not contain smallpox virus and cannot cause smallpox disease," says Dr. Joanne Cono, a medical epidemiologist involved with the Bioterrorism Preparedness and Response Program at the CDC. The distinction between vaccinia and smallpox is important in considering the potential risks of a large-scale vaccination program. Vaccination can lead to transmission of the vaccinia virus, but as the CDC states on its website, there is no chance of a smallpox outbreak due to the government's program.
Despite the fact that the vaccination program cannot start a smallpox outbreak, the risk of side effects from the vaccinia virus is not trivial. The potential benefits of vaccination need to be weighed against the possible side effects. Since the population of the United States is nearly 300 million people, a mass vaccination strategy targeting the entire population could lead to serious side effects in thousands of people. Cono believes that "at this time, when there is no smallpox disease on the planet, the risks of vaccination outweigh the potential benefits for the general public, but in the event of an outbreak, the benefits would outweigh the risks for people who are at high risk for exposure to smallpox." In the past, when smallpox was prevalent, the potential benefits of mass-vaccination did outweigh the risks. Now, since smallpox has been eradicated from the world, health officials do not believe it would be wise to vaccinate the general public because of the potential side effects.
In preparing for the possibility that smallpox might be reintroduced through a bioterrorist attack, the government has decided to vaccinate "first responders," those public health and medical professionals who would treat and investigate the first occurrences of smallpox. In addition, the vaccination program also targets military personnel "who are or may be deployed in high threat areas."
Vaccinating healthcare workers now will facilitate a response to any future smallpox outbreak since vaccinated workers will be able to treat patients without risk of contracting the disease themselves or communicating it to others. "In the event of an outbreak, other healthcare workers would be vaccinated so the work could grow substantially," says Cono. Furthermore, vaccinating additional workers would allow healthcare centers to treat more patients in the case of a large outbreak.
The smallpox vaccination program is only one step in the broader plan to respond to a potential smallpox outbreak. "There are many steps in stopping an outbreak," Cono says. "Vaccination of public health and medical responders before an outbreak just helps us prepare. During an outbreak the other steps include identifying and isolating those with smallpox so that they do not spread the disease, identifying and vaccinating the people they contact to minimize the risk that they will contract smallpox (this is called ring vaccination), and if necessary, open large-scale vaccination clinics to offer smallpox vaccine to the general public in the affected geographic areas."
By implementing the ring vaccination strategy, smallpox can be tamed before it gets a chance to spread. Every time a person is diagnosed with smallpox, a ring of people who come into close contact with that person are also vaccinated. This action helps prevents infection in those contacts on an individual level and also creates a buffer zone to prevent the spread of smallpox in the general population. Like the smallpox vaccine, the ring vaccination strategy is not new; it was used to eradicate smallpox from the world in the 1970s. Its importance to the overall smallpox response plan is summarized in a CDC statement, which says, "any vaccination strategy for containing a smallpox outbreak should use the ring vaccination concept."
Using the ring vaccination strategy also means that the general public could avoid vaccination even if some people start contracting smallpox. "Even in an outbreak, not everyone would need to get vaccinated," Cono says. With the ring strategy, she says, "there is always the potential for large-scale vaccination, but the main goal is to ensure that family members and close contacts get vaccinated as quickly as possible."
According to a CDC website, 37,915 healthcare workers had been vaccinated against smallpox as of July 11, 2003. As of May 2003, the CDC reports that only one vaccinee had developed a potentially life-threatening side effect. However, 45 have developed moderate side effects, which are classified as treatable but require healthcare visits, or severe side effects, which result in hospitalization. These events underscore the principle behind the smallpox vaccination policy: to balance the bioterrorist smallpox threat and the side effects of the vaccine, only those first-responders who would be essential to containing a smallpox outbreak are being vaccinated.
Suggested Reading
Centers for Disease Control. Protecting Americans: Smallpox Vaccination Program. 13 December 2002. Last Accessed: 29 July 2003. < http://www.bt.cdc.gov/agent/smallpox/vaccination/vaccination-program-statement.asp>
Centers for Disease Control. Smallpox. 17 July 2003. Last Accessed: 22 July 2003. < http://www.bt.cdc.gov/agent/smallpox/>
Centers for Disease Control. Smallpox Response Plan. 30 June 2003. Last Accessed: 22 July 2003.
Centers for Disease Control. Smallpox Vaccination Adverse Events Report. 11 July 2003. Last Accessed: 22 July 2003.
Cohen, J. and M. Enserink. Rough-and-Tumble Behind Bush's Smallpox Policy. Science. 298: 2316-2319 (2002).
Fauci, A.S. N. Engl. J. Med. 346: 1319 (2002)
Food and Drug Administration. Dryvax Vaccine. Last Accessed: 22 July 2003.
Halloran, M.E., et.al. Containing Bioterrorist Smallpox. Science. 298: 1428-1432 (2002)