Author: António J. P. Pagarete
Institution: University of the Azores, Portugal
Date: May 2005
Abstract
In the Azorean Archipelago, the limpet Patella candei gomesii Drouët (1858) exhibits two distinct habitat morphs with respect to shell morphology: mosca, which is highly conical and commonly found above high tide mark, and mansa, which is flatter and intertidal. Although both morphs occur microsympatrically, their distribution is not random. This paper examines the genetic diversity between the two morphs over a set of 10 enzyme loci. Specimens of both forms were sampled on the two most oriental islands of this archipelago. High genetic identity values were found, thus rejecting the notion of reproductive isolation within these populations or between both habitat morphs. These results support the conspecifity of the two forms, and argue in favour of this being yet another example of morphological plasticity among limpets.
Figure 1. Shells of Patella candei gomesii (A-B) mansa and (C-F) mosca.
Introduction
Habitat heterogeneity has for long been supposed to increase genetic variation (Hedrick 1986; Hedrick et al. 1976). Theoretical models (e.g., Levene 1953; Maynard Smith and Hoekstra 1980) and laboratory experiments (Kassen 2002) are consistent with the existence of associations between habitat heterogeneity and species diversity, as well as with intra- and inter-populational variation. Models of ecological speciation further emphasise that ecological mechanisms are indeed forceful enough to drive speciation (Schluter 2001). The spatial scale over which genetic differentiation among populations occurs is important because it reflects the action of ecological processes, such as dispersal, against genetic processes, such as gene flow, genetic drift, and selection (Van der Strate et al. 2003).
Rocky shores are extreme environments with respect to physical heterogeneity, due to sharp gradients in abiotic variables such as temperature, salinity, wave action, and irradiation. These are associated with sharp transitions in organism distribution (Johannesson 2003). Gastropod species of heterogenous environments are particularly suited to develop phenotypic polymorphic adaptations due to their restrained mobility (Côrte-Real et al. 1992; Boulding and Hay 1993; De wolf et al. 2000; Johannesson 2003).
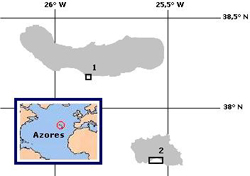
Figure 2. Map of the oriental group of the Azores Archipelago showing sampling sites at (1) Agua dAlto (Sao Miguel Island) and (2) Praia Formosa (Santa Maria Island).
The taxonomic status of the Macaronesian limpet Patella candei is not clear (Titselaar 1998). Recent studies indicate that the populations inhabiting the Azorean coasts have phyletically evolved from the populations from Madeira Archipelago into the current Azorean P.c. gomesii (Weber and Hawkins 2002). However, contradicting genetic evidence is not the only factor hampering the clarification of P. c. gomesii. Extreme variations in this species' shell morphology has also led to confusion over the classification of its possible ecotypes (Titselaar 1998). Specimens fall into two distinct varieties (Figure 1): mosca - highly conical and commonly found above high tide mark, and mansa flatter than mosca and mostly intertidal (Hawkins et al. 1990). Recent morphometric measurements have proven that both shell height and radula parameters distinguish mosca and mansa habitat morphs, thus supporting morphological variation within P.c. gomesii (Curdia 2004).
Table 2: Name, EC number, abbreviation, number of loci, and electrophoretic conditions for each enzymatic system analyzed. a: Buffer system compositions: 1: Citrate - NaOH-His/Hcl pH 6.0 (Ferrand and Amorim 1990). 2: Tris - NaH2PO4 pH 7.6 (Branco et al. 1999). 3: Tris-citrate pH 7.6 (Amorim and Siebert 1982). IUBNC (1984).
The morphological plasticity found in this species may result from ecological factors, and is thus called ecophenotypical variation. This plasticity is common in gastropoda (e.g., De Wolf et al. 1998; Côrte-Real et al. 1992; Titselaar 1998; Johannesson 2003). Enzyme electrophoresis has been used successfully in the past to discriminate cryptic species (e.g., Backeljau et al. 1994), establish levels of intraspecific variation (e.g., Thorpe 1983; Weber and Hawkins 2002), and identify stocks for management purposes (e.g., Smith et al. 1990; Weber et al. 1998). Tracing variation in ecophenotypes to genetic levels has led to contradictory results. For example, allele frequencies have been found to parallel environmental gradients of salinity and temperature (Johannesson & Johannesson 1989), whereas very high genetic identities have been described by Côrte-Real, et al. (1992) for different morphotypes of Patella aspera, a sympatric limpet in the Azores.
This work focuses on the taxonomic uncertainty in P.c. gomesii, addressing the causes behind its morphological plasticity. Specimens of the two morphotypes were obtained from both extreme conditions (i.e., inter-tidal and above-wave intervention), and their allozyme patterns were determined to assess the level of genetic exchange between P.c. gomesii populations and morphotypes. This study of the association of Patella candei gomesii morphological plasticity with genetic variation is a contribution to a wider ongoing project which aims to describe and study the phylogeography of North Atlantic limpets.
Methods & Materials
Collection of samples
Table 3. Percentage of polymorphic loci and allele frequencies per locus and overall loci. a Mean observed heterozygosity (direct-count). b Mean unbiased heterozygosity based on Hardy-Weinberg expectation. c Percentage of polymorphic loci: a locus is considered polymorphic if the frequency of the most common allele does not exceed 0.99. Key: P (HDW) Probability test for Hardy-Weinberg equilibrium departure for each population, assuming _ = 0.05, based on Fishers method; (n) sample size
Specimens of Patella candei gomesii were handpicked on São Miguel and Santa Maria islands in the Azores Archipelago (Figure 2). Both forms were sampled in October 2002 and January 2004 from Água d'Alto (São Miguel), and from Praia Formosa (Santa Maria) in May 2003 (see Table 1 for details). Habitat morphs were identified using the shell characteristics outlined previously (Hawkins et al. 1990). Specimens recognized as mansa and mosca were obtained, respectively, from the intertidal and above the high tide mark. The animals were transported live to the laboratory, where the foot muscle was dissected and stored at 80ºC.
Electrophoresis
Homogenates were prepared by sonication of thawed foot muscle and centrifuged at 14000 rpm for 15 min and at 4ºC. Before electrophoresis, supernatants (with exception for IDH enzyme) were incubated in 10:3 DTT solution (120 _M) at 37ºC for 1 hour. Standard horizontal 15% starch (Biomol Chemicals, Hamburg) gel electrophoresis was carried out using the conditions that apply to each enzymatic system (Alexandra Sá Pinto, pers. com.) (see Table 2). Staining procedures followed modified techniques of Harris & Hopkinson (1976). Typed specimens were included with each run in order to compare different gels.
Table 4. Observed and expected heterozygosities (Ho and He respectively) and exact probability tests for overall loci Hardy-Weinberg equilibrium departure in mixed sampled populations, assuming _ = 0.05, based on Fishers method. Key: AA = Agua dAlto, PF = Praia Formosa.
Genepop 3.4 (Raymond & Rousset 2003), the population genetics software package, was used to calculate allele frequencies, genic differentiation between each population, and probability tests for Hardy-Weinberg equilibrium departure for each sampled form and for each mixed group: joining both mosca samples (mixed mosca), both mansa samples (mixed mansa), all specimens from Água d'Alto (mixed AA), all specimens from Praia Formosa (mixed PF), and all the sampled individuals (overall). These probability tests were based on Fisher's method and were further corrected for multiple comparisons using the Sequential Bonferroni method (Rice 1989). Mean observed heterozygosity and mean expected unbiased heterozygosity based on Hardy-Weinberg equilibrium were calculated with the Genetix v.4.05 (Belkhir et al. 2004) population genetics software package. Allelic richness of each population was calculated with FSTAT 2.9.3 (Goudet 2001).
The Gendist program, included in the Phylip software package (Felsenstein 1989), was used to compare the sampled forms by Nei's (1972) and Cavalli-Sforza & Edwards (1967) unbiased genetic distances.
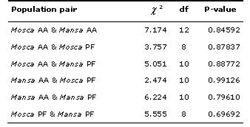
Table 5. Genic differentiation between each population pair based on Fishers method. Key: AA = Agua dAlto; PF = Praia Formosa; df = degrees of freedom.
Results
Ten loci were resolved for both habitat morphs from the two islands. Allele frequencies, variability measures, probability tests for Hardy-Weinberg equilibrium departure, and F-statistics results are summarized in Table 3. Four loci were consistently monomorphic for all populations (IDH, MDH, MPI, and PEP D). Low values of polymorphism were registered, ranging from 20% (mansa, Praia Formosa) to 50% (mansa, Água d'Alto). PGM-1 and PGM-2 were the most polymorphic loci. These values were relatively low when compared to the 58.33% described previously for this species (Côrte-Real et al. 1996). With the exceptions of these two loci, there were few differences between the four groups regarding the frequencies of the most common allele in polymorphic loci. The values of allelic richness of the four groups were very close (1.309-1.719).
Table 6. Unbiased genetic distances between populations. Above diagonal: Neis (1972). Below diagonal: Cavalli-Sforza & Edwards (1967). Key: AA = Agua dAlto; PF = Praia Formosa.
All the significant departures from Hardy-Weinberg equilibrium registered (see Tables 3 and 4) were no longer significant after sequential Bonferroni correction for multiple comparisons (Rice 1989). HO and He values for each sampled form and within each mixed group differed only slightly (Table 4). No significant genic differentiation was registered between each population pair (Table 5). This is reflected in the very low values of unbiased genetic distances between populations (Nei's 1972; Cavalli-Sforza & Edwards 1967) presented in Table 6.
Discussion
Enzyme electrophoresis was used to probe the genetic basis of the two morphotypes of P. candei (mosca and mansa) from the two Azorean islands populations. The allelic frequencies, heterozygosities, and allelic richness of the sampled populations showed comparable values, arguing against the existence of a clear differentiating pattern between habitat morphs.
Data also point to the conclusion that the two morphotypes are not reproductively isolated. Given the nonsignificant departure associated with Hardy-Weinberg equilibrium, the four samples are likely panmitic. Furthermore, the values for deviation between HO and He in the sampled populations (Table 3) were similar to those obtained when the specimens were grouped either by morphotype or by island (Table 4). The genetic distances (Nei 1972) found between the four groups (Table 6) are within the range considered by several authors (i.e. 0.0 0.1; Ayala et al. 1974; Côrte-Real et al. 1992, 1996; Thorpe 1983; Nei 1987) to be typical of conspecific comparisons. All considered, both mosca and mansa forms of P. candei gomesii probably represent the same breeding unit.
These results are consistent with the synchronous timing of P. candei gomesii's reproductive cycle found in a recent study (Cúrdia, pers. com.). This is the second study that concludes that there exist ecologically related morphotypes on Atlantic limpets of the genus Patella. Two subtidal Patella aspera morphs with very high genetic identities have also been described by Côrte-Real, et al. (1992). Together they point to the existence of morphological plasticity in the Patella genus. On a more general level, this new data adds to the concept of ecophenotypical variation, which suggests that the morphology of an organism is largely influenced by environmental conditions (Johannesson 2003). Evidence of ecophenotypes have been uncovered in various gastropods (e.g., De Wolf et al. 1998; Titselaar 1998), particularly related to gradients of exposure to wave action and tidal height (Côrte-Real et al. 1992).
The steady decline reported for P. candei gomesii (Ferraz et al. 2001) reinforces the importance of clarifying its taxonomic status. This work did not find traces of a genetic basis in support of the morphological variability of P. candei gomesii. An increase in the number of polymorphic loci analysed, or in the diversity of genotypes scanned for each locus, could challenge this conclusion. An extended multigenotyping survey throughout other islands of the archipelago would be the recommended for the study of this species.
Acknowledgements
This study is part of the project "Species from the genus Patella as a model for the study of evolutionary patterns and dynamics of marine organisms" (POCTI/BSE/42003/2001), funded by Fundação para a Ciência e Tecnologia (FCT, Portugal). We thank the CIBIO (Research Center in Biodiversity and Genetic Resources, University of Porto) for their guidance and support, especially Dra. Alexandra Sá Pinto and Professor Dr. Paulo Alexandrino; Dra. Amélia Fonseca for the support and facilities provided; Dr. João Cúrdia for the collecting of samples, help in the laboratory, and for useful discussions and comments.
References
Amorim A and G Siebert. (1982). Glutamate pyruvate transaminase, esterase D, glyoxalase I, and phosphoglucomutase 1 polymorphisms in Porto district (Portugal). Hum Hered. 32:293-300.
Ayala FJ, Tracey ML, Hedgecock D, et al. (1974). Genetic differentiation during the speciation process. Drosophila Evolution. 28:576-92.
Backeljau T, Bouchet P, Gofas S, et al. (1994). Genetic variation, systematics and distribution of the venerid clam Chamelea gallina. J Mar Biol Ass UK. 74:211-23.
Belkhir K, Borsa P, Chikhi L, et al. (2004). GENETIX 4.05, Population genetics software for Windows TM. Université de Montpellier II. Montpellier.
Boulding EG and TK Hay. (1993). Quantitative Genetics of Shell Form of an Intertidal Snail: Constraints on Short-Term Response to Selection. Evolution. 47(2):576-92.
Branco M, Machado JC, and N Ferrand. (1999). Extensive genetic polymorphism of peptidases A, B, C, and D, in wild rabbit (Oryctolagus cuniculus) populations from the lberian Peninsula. Biochem Genet. 37:237-49.
Cavalli-Sforza LL and AWF Edwards. (1967). Phylogenetic analysis: models and estimation procedures. Evolution. 32:550-570.
Côrte-Real HBSM, Hawkins SJ, and JP Thorpe. (1992). Genetic confirmation that intertidal and subtidal morphs of Patella ulyssiponensis aspera Roding (Mollusca: Gastropoda: Patellidae) are conspecific. Arquipélago. 10:55-66.
Côrte-Real HBSM, Hawkins SJ, and JP Thorpe. (1996). Population differentiation and taxonomic status of the exploited limpet Patella candei in the Macaronesian islands (Azores, Madeira, Canaries). Marine Biology. 125:141-52.
Curdia J. (2004). personal communication.
De Wolf H, Backeljau T, and R Verhagen. (1998). Spatio-temporal genetic structure and gene flow between two distinct shell morphs of the planktonic developing periwinkle Littorina striata (Mollusca: Prosobranchia). Mar Ecol Prog Ser. 163:155-63.
De Wolf H, Verhagen R, and T Backeljau. (2000). Large scale population structure and gene flow in the planktonic developing periwinkle, Littorina striata, in Macaronesia (Mollusca: Gastropoda). J Exp Mar Bio and Eco. 246:69-83.
Felsenstein J. (1989). PHYLIP, Phylogeny Inference Package (Version 3.2). Cladistics. 5:164-6.
Ferrand N and A Amorim. (1990). Genetic polymorphism of _-aminolaevulinic acid dehydratase (E.C. 4.2.1.24, ALAD) in the domestic rabbit. Anim Genet. 21:217-9.
Ferraz RR, Menezes GM, and RS Santos. (2001). Limpet (Patella spp.) exploitation in the Azores, during the period 1993-1998. Arquipélago, Life and Marine Sciences. Supplement 2 (Part B):57-63.
Goudet J. (2001). FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9.3). Available from http://www.unil.ch/izea/softwares/fstat.html. Updated from Goudet, J. (1995). FSTAT v-1.2. A computer program to calculate F-statistics. J Heredity. 86:485-6.
Harris H and DA Hopkinson. (1976). Handbook of Enzyme Electrophoresis in Human Genetics. American Elsevier. New York.
Hawkins SJ, HBSM Côrte-Real, HR Martins, et al. (1990). A note on the identity of Patella in the Azores. Açoreana. Supplement:167-73.
Hedrick PW. (1986). Genetic polymorphism in heterogeneous environments: a decade later. Ann. Rev. Ecol Syst. 17:53566.
Hedrick PW, Ginevan ME, and EP Ewing. (1976). Genetic polymorphism in heterogeneous environments. Ann Rev Ecol. Syst. 7:132.
IUBNC (International Union of Biochemistry, Nomenclature Committee). (1984). Enzyme nomenclature. Academic Press. San Diego.
Johannesson K. (2003). Evolution in Littorina: ecology matters. J Sea Res. 49:107-17.
Johannesson K and B Johannesson. (1989). Differences in allele frequencies of Aat between high- and mid-rocky populations of Littorina saxatilis (Olivi) suggest selection in this enzyme locus. Genet Res Camb. 54:7-11.
Kassen R. (2002). The experimental evolution of specialists, generalists, and the maintenance of diversity. J Evol Biol. 15:17390.
Levene H. (1953). Genetic equilibrium when more than one ecological niche is available. Am Nat. 87:3313.
Maynard Smith J and R Hoekstra. (1980). Polymorphism in a varied environment: How robust are the models? Genet Res Camb. 35:4557.
Nei M. (1972). Genetic distance between populations. American Naturalist. 106:283-92.
Nei M. (1987). Genetic distance and molecular phylogeny. Pp. 193-223 in: Ryman, N., F. Utter (Eds.) Population Genetics and Fishery Management. Seattle. USA.
Raymond M and F Rousset. (2003). Genepop 3.4., an updated version of Genepop V.1.2 (1995): populationgenetics software for exact tests and ecumenicism. J Heredity. 86:248-9.
Rice WR. (1989). Analyzing tables of statistical tests. Evolution. 43:223-5.
Schluter D. (2001). Ecology and the origin of species. Trend Ecol Evol. 16:37280.
Smith PJ, Jamieson A, and AJ Birley. (1990). Electrophoretic studies and the stock concept in marine teleosts. Journal du Conseil International pour l'Exploration de la Mer. 47:231-45.
Titselaar FFLM. (1998). A revision of the recent European Patellidae (Mollusca: Gastropoda). Part 1. The Patellidae of the Azores, Madeira, the Selvagens and the Canary Islands. Vita Marina. 45(3-4):21-62.
Thorpe JP. (1983). Enzyme variation, genetic distance and evolutionary divergence in relation to levels of taxonomic separation. Pp. 131-152 in: OXFORD, G.S., D. Rollinson (Eds). Protein Polymorphism: Adaptive and Taxonomic Significance. Academic Press. London.
Van der Strate HJ, Van de Zande L, and Stam WT, et al. (2003). Within-island differentiation and between-island homogeneity: non-equilibrium population structure in the seaweed Cladophoropsis membranacea (Chlorophyta) in the Canary Islands. European J Phycology. 38:15-23.
Weber LI and SJ Hawkins. (2002). Evolution of the limpet Patella candei d'Orbigny (Mollusca, Patellidae) in Atlantic archipelagos: human intervention and natural processes. Biological J Linnean Society. 77:34153.
Weber LI, Thorpe JP, Santos RS, et al. (1998). Identification of stocks of the exploited limpets Patella aspera and P. candei at Madeira archipelago by allozyme electrophoresis. J Shellfish Research. 17(4):945-53.