Author: Lovejoy Katherine
Institution: Chemistry and Integrated Science
Date: August 2002
It is a common sight at airports: a planeload of people sitting and waiting for their delayed flight. The passengers are set to go, but an inadequacy in the transport system has stalled travel. A similar transportation problem has developed since the sequencing of the human genome. Although some newly-sequenced genes may help cure genetic diseases by altering the genetic program of the cells, a treatment called gene therapy, there is still no reliable, completely successful method to transport these reparative genes into disease-causing cells.
article_480_order_0
Gene therapy treats diseases of genetic origin by providing the missing genetic material, by correcting defective genes, or by increasing the expression of genes already present (Friedmann 1998). The transportation problem is difficult because it depends on the number of genes that need to be inserted and their cellular destination, each of which is disease specific.
Some gene therapies can use non-specific transportation-the genes can simply be loaded onto the therapeutic equivalent of a jumbo jet headed for a big city, like Chicago. The "passenger" genes are added to the bloodstream and scatter as they go about their business. Other genes require a more elaborate and personalized chartered flight that takes them right to the doorstep of the targeted cells, helps them cross the cell membrane and then locate the nucleus, where the genes instruct the cell to create beneficial proteins. This process is called transfection and can be achieved in several different ways.
Viral vectors are the most advanced of current transfection technologies and are currently being tested in human subjects. Viral transfection involves the insertion of therapeutic DNA into the viral genome. The virus then is administered to and infects the patient and, ideally, delivers the precious cargo to a diseased cell nucleus.
The first retroviral vectors were made from the murine leukemia virus and carried their genetic passengers into dividing cells. The therapy was of limited value, however, because most of the body's cells are non-dividing. Adenoviruses (Figure 1), the type of viruses that cause the common cold, had a higher rate of delivery, but the immune system soon kicked the foreign material out of the body (Smith 1995).
article_480_order_1
One family of viral vectors that is currently of great interest is the lentivirus family, the members of which can incorporate their passenger genes into nondividing cells. A drawback of lentivirus vectors is the fact that they are made from deadly viruses, such as HIV and Ebola, and some scientists are skeptical of putting them to use for therapeutic purposes.
Perhaps the most promising viruses are the adeno-associated viruses (AAVs), which cause no known disease in humans and hold promise for long-term expression. But even with this most promising system, there is a hurdle for scientists to overcome because AAVs have proven difficult to produce in mass quantities (Smaglik 1998).
article_480_order_2
Viral transfection is the method that most often comes to mind when many people think of gene therapy, because it is the one most often in the news. A prominent example is the 1999 case of Jesse Gelsinger, a patient who died from inflammation and other complications associated with an adenoviral therapy experiment (Weiss 1999). Public attention suddenly focused on the safety of viral transfection. The problems with inflammation, producing viral vectors in mass quantities, and public discomfort with the use of viruses for therapeutic purposes led to increased interest in the development of non-viral transfection vectors.
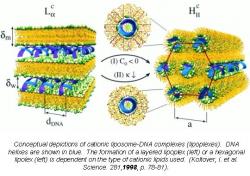
An alternative to viral therapies are cationic lipid transfection systems (Felgner 1987). These lipid systems feature molecules, such as the one in Figure 2, that are composed of three parts: a hydrophobic anchor, a linker, and a head group (Figure 3). The hydrophobic end of the molecule avoids contact with water, while the positively-charged head group seeks contact with water. In order to protect their hydrophobic end from water and expose the hydrophilic ends, the molecules self-assemble in water to form micelles (Figure 4). When DNA, with its natural negative charge, is introduced to these positively-charged micelles, compound structures called lipoplexes are formed. The lipoplex mostly or totally protects the DNA from the outside environment and is taken into the cell (Koltover 1998).
Cationic liposome transfection systems are promising tools for gene therapy. They are minimally toxic to the organism receiving the therapy and are able to handle larger amounts of DNA than viral vectors (Miller 1997) - they are jumbo jets, not puddle jumpers. Another problem that cationic lipids may solve is the difficulty of targeting the therapeutic DNA to the specific organ or part of the body that is ailing. For example, DNA designed to treat cancerous cells would ideally be attracted specifically to cancer cells for maximum therapeutic value (Schatzlein 2001).
Cationic lipids can potentially be targeted to specific cell types by incorporating certain ligands into their structure. These ligands would bind cell-surface receptors uniquely expressed on the target cells and would help ensure that the therapeutic DNA finds the disease-causing cells. Incorporating such ligands into cationic liposomes is an area of high research interest.
Other research in the field of non-viral vectors involves maximizing transfection efficiency, which is a weak point in liposome transfection systems (Polymer-based. 2001). One explanation for poor transfection efficiency could be the patient's immune responses against the cationic lipids, while another could be the fact that many cationic vectors are unstable in the presence of serum. Serum can be removed from test tubes during in vitro experiments, but not in living patients, so current research is also focusing on investigating synthetic vectors that can tolerate serum (Chesnoy 2000).
article_480_order_3
Many transfection systems, both viral and non-viral, seem promising in the lab, yet fail when introduced into humans, so the enormous number of DNA transportation systems that are being tested today may yield only a few viable treatments for gene therapy. Despite the negative press that these failures in gene therapy research have recently drawn, the field is an active one, with government, academic, and private labs all working toward the magic bullet that will reliably deliver therapeutic genes to malfunctioning human cells.
Suggested Reading
Chesnoy, S. and L. Huang. "Structure and Function of Lipid-DNA Complexes for Gene Delivery." Annu. Rev. Biophys. Biomol. Struct. 29 (2000): 27-47.
Polymer-based Vectors. Gene Delivery Group, University of Birmingham. 26 Jan. 2001 http://web.bham.ac.uk/can4psd4/nonviral/polymer.html.
Felgner, P.L. et al. "Lipofection-A Highly Efficient, Lipid-Mediated DNA-Transfection Procedure." Proc. Natl. Acad. Sci. 84 (1987): 7413-7414.
Friedmann, T., Ed. The Development of Gene Therapy. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 1998.
Koltover, I. et al. "An Inverted Hexagonal Phase of Cationic Liposome-DNA Complexes Related to DNA Release and Delivery." Science. 281(1998): 78-81.
Miller, A. "Cationic Liposomes for Gene Therapy." Angew. Chem. Int. Ed. 37(1997): 1768-1785.
Schatzlein, A.G. "Non-viral Vectors in Cancer Gene Therapy." Anti Cancer Drugs. 12 (2001): 275-304.
Smaglik, P. "Gene Therapy - The Next Generation." The Scientist 12 [10}: 4 (1998) 26 Jan. 2001 http://www.the-scientist.com/yr1998/may/smaglik_p4_980511.html.
Smith, A. E. . "Viral vectors in gene therapy." Annual Review of Microbiology 49(1995): 807-838.
Weiss, R. and D. Neldson. "Teen Dies Undergoing Gene Therapy." Washington Post. 29 Sept. 1999: A1.