Author: Condliffe Elizabeth
Institution: Mechanical Engineering
Date: April 2002
This article is Part One of a two-part series examining alternative energy sources.
Producing energy with water as the only by-product is the attractive concept behind fuel cells. They function by combining hydrogen with oxygen - a simple reaction. Oxygen occurs in air, but highly-reactive hydrogen does not exist naturally in isolation. Current fuel cell research focuses on finding appropriate hydrogen sources and methods for accomplishing this reaction.
The United States was the first nation to actually use fuel cells, powering the Gemini and Apollo spacecraft with them in the 1960s. However, the first fuel cell was built long ago - in 1839 - by Sir William Grove, a Welsh judge and scientist. Fuel cells are used in many different commercial and industrial applications. They function as stationary power sources at landfills and wastewater treatment facilities, reducing methane emissions while producing power for the surrounding area. Buses in Vancouver and a few other cities are powered by fuel cells, emitting steam, rather than greenhouse gases, as exhaust.
In the future, researchers hope to develop cars powered by fuel cells, fuel cell battery replacements, stand-alone fuel cell generators for households and many other specific applications. The intensity of research was recently increased when the Bush administration announced a new fuel cell initiative with the automobile industry entitled FreedomCAR (Cooperative Auto Research) to develop emission- and petroleum-free cars and light trucks.
Political and Environmental Motivations
Fuel cells may provide a viable alternative to burning fossil fuels. For both environmental and national security reasons, our dependence on oil needs to be reduced. We must reduce not only our use of non-renewable resources, but also the emissions associated with combustion. Fossil fuel combustion generates greenhouse gases such as carbon dioxide, as well as harmful photochemical gases that combine nitrogen or sulfur and oxygen (NOX and SOX).
The need for an energy revolution is not only environmental. Our high oil requirements have to be considered when generating foreign policy. In a recent article about gas mileage, Robert F. Kennedy Jr. wrote, "maintaining our national security will require reducing our dependence on foreign oil."
How Fuel Cells Work
article_468_order_0
The basic fuel cell combines hydrogen gas with oxygen to produce energy in the form of electricity, with water and heat as the only byproducts.
H2 + 1/2 O2 --> H2O + electricity + heat
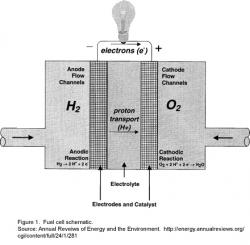
Figure 1 shows a schematic of this reaction in a fuel cell. Most types of fuel cells split hydrogen into two protons and two electrons at a catalytic anode (a negatively-charged plate that splits hydrogen gas (H2) without any change to the plate). The protons flow through a solid electrolyte, which is impermeable to the electrons, to the cathode (positively charged plate). The electrons flow through an external circuit, generating an electrical current. At the cathode, the electrons and protons from the hydrogen combine with oxygen to form water. It is the current through the external circuit that provides the usable electricity.
The laws of chemistry limit an ideal fuel cell to generating 1.229 Volts; actual achieved voltages per fuel cell are less than 1 Volt. Yet, standard North American outlets are 110 Volts. To obtain greater voltages, multiple cells are stacked in series. All practical applications use stacks, usually containing between 80-100 cells in series.
Sources of Hydrogen
Because hydrogen is an extremely explosive gas, it must be stored at atmospheric pressure if it is to be supplied directly to the fuel cell. In addition, the system must not have any leaks and must not require any flames.
article_468_order_1
Rather than requiring a supply of hydrogen, all commercial applications use a reformer to isolate hydrogen gas from fossil fuels such as methanol, ethanol, and natural gas. Although these are the same fuels that traditional combustion power plants use, reformers coupled with fuel cells make a much more efficient system, producing more usable energy from the same amount of fossil fuel. In addition, the only byproduct of the reforming process is carbon dioxide, whereas combustion produces harmful nitrogen oxides (NOX) and sulfur oxides (SOX).
Stationary systems consist of a reformer to isolate hydrogen from the fuel, followed by a fuel cell stack and then a power conditioner that uses the "waste" heat to heat water. They may also become de facto space heaters, giving off waste heat. Stationary systems can reach an overall efficiency greater than 80%, far greater than any combustion power generators despite using the same initial fuels.
Theoretically it is possible to produce hydrogen gas by electrolyzing water (splitting it into its hydrogen and oxygen components). Regenerative fuel cells will do this using solar or wind energy accompanied by catalytic enzymes in bacteria and algae. As with other fuel cells, hydrogen would then be channeled to the anode and the oxygen to the cathode. However, such regenerative fuel cells remain a dream for fuel cell researchers, as they have yet to be commercially manufactured.
Types of Fuel Cells
Using the same principle of generating energy by combining hydrogen with oxygen to form water, different types of fuel cells strive for reliability, efficiency, and affordability. Generally, the variations change the membrane that separates the hydrogen and oxygen and is permeable only to the protons.
PEM Fuel Cells
The basic fuel cell is a Proton Emission Membrane (PEM) fuel cell. The membrane separating the hydrogen and oxygen is a solid electrolyte generally about 125 mm thick. PEMs, which operate below 100OC, are easy to use, have high power densities and have the ability to vary their energy outputs quickly. However, for the membrane to function effectively, it needs a high humidity that is a challenge to maintain for transportation applications.
Alkaline Fuel Cells
article_468_order_2
Alkaline fuel cells were originally researched and used by NASA. They use a concentrated alkaline solution of potassium hydroxide as the electrolyte, operate between 120-250 OC and reach an efficiency slightly less than 70%. These cells are extremely expensive.
Solid Oxide Fuel Cells
Solid oxide fuel cells use another variety of electrolyte. In this case, a hard ceramic electrolyte is used, but to be conductive, these ceramics require the operating temperature to be 600-1000OC. As a result, solid oxide fuel cells are only practical for stationary power plants.
Direct Methanol Fuel Cell
The direct methanol fuel cell is very different from other fuel cells. It eliminates the need for a fuel reformer while using the same thin solid electrolyte as PEM fuel cells. The anode catalyst draws the hydrogen protons directly from liquid methanol. This process is slower, but the same amount of energy is produced. Direct methanol fuel cells are being tested as a substitute or replacement for batteries. Instead of replacing the battery, one would simply replace a refillable methanol cartridge. These fuel cells would operate at temperatures as low as 45OC, but have an efficiency cost reducing the overall efficiency to 40%.
Since the Apollo missions, researchers have been working to perfect fuel cells. The theory is extremely promising and the fuel cell power systems currently functioning are producing efficient energy. However, the costs involved with fuel cell technology are a limiting factor.
Already there are more than 200 stationary fuel cell units in place in public buildings. As technology improves, and the associated costs decrease, fuel cells should be one of the keys to reducing our foreign oil dependence while reducing harmful emissions.
Suggested Reading
For further information on fuel cells, visit:
http://www.fuelcells.org
http://www.eren.doe.gov/RE/hydrogen.html
http://www.fuelcelltoday.com
http://www.princeton.edu/~abbgroup/fuel.html
Or, for industry information, visit:
http://www.hpower.com
http://www.internationalfuelcells.com
http://www.ballard.com